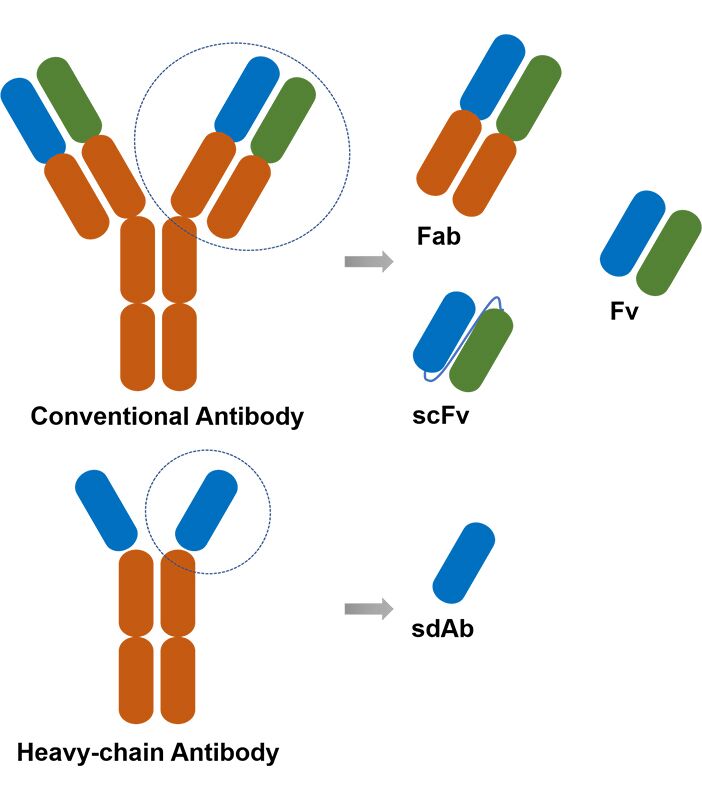
In most cancer diseases, cell surface molecules composed of membrane proteins are often important diagnostic and therapeutic targets. Membrane proteins have complex structures and diverse functions, and play an important role in many physiological processes in the human body. In this case, antibodies against membrane proteins play a key role in diagnostic and therapeutic drug development or theoretical basic research.
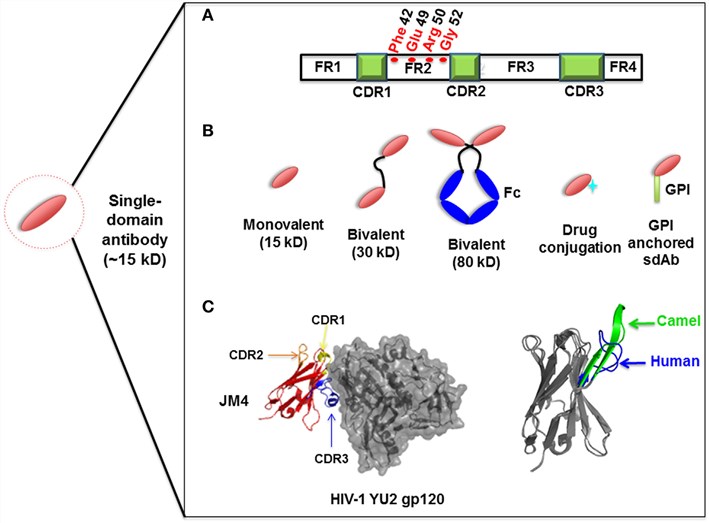
Due to many limitations, the development of antibodies against membrane protein targets is extremely challenging. Creative Biolabs' solution is to use the novel VHH instead of full-length IgG or other antibody fragments. VHH has the advantages of small size, high stability, and strong penetration, which can recognize the most hidden epitopes, including membrane protein targets. At the same time, our scientists also integrate the Magic™ membrane protein platform and DNA immunization strategy into our service process to provide a comprehensive and one-stop antibody development solution.
For this case study, the mixed antigens, which include 3 different targets, were used as antigens and screening targets. Creative Biolabs is entrusted to immunize 1 camelid host animal and then develop antigen-specific single domain antibodies, respectively. Finally, 7 unique antigen-1-specific sdAbs, 5 unique antigen-2-specific sdAbs, and 19 unique antigen-3-specific sdAbs were obtained.
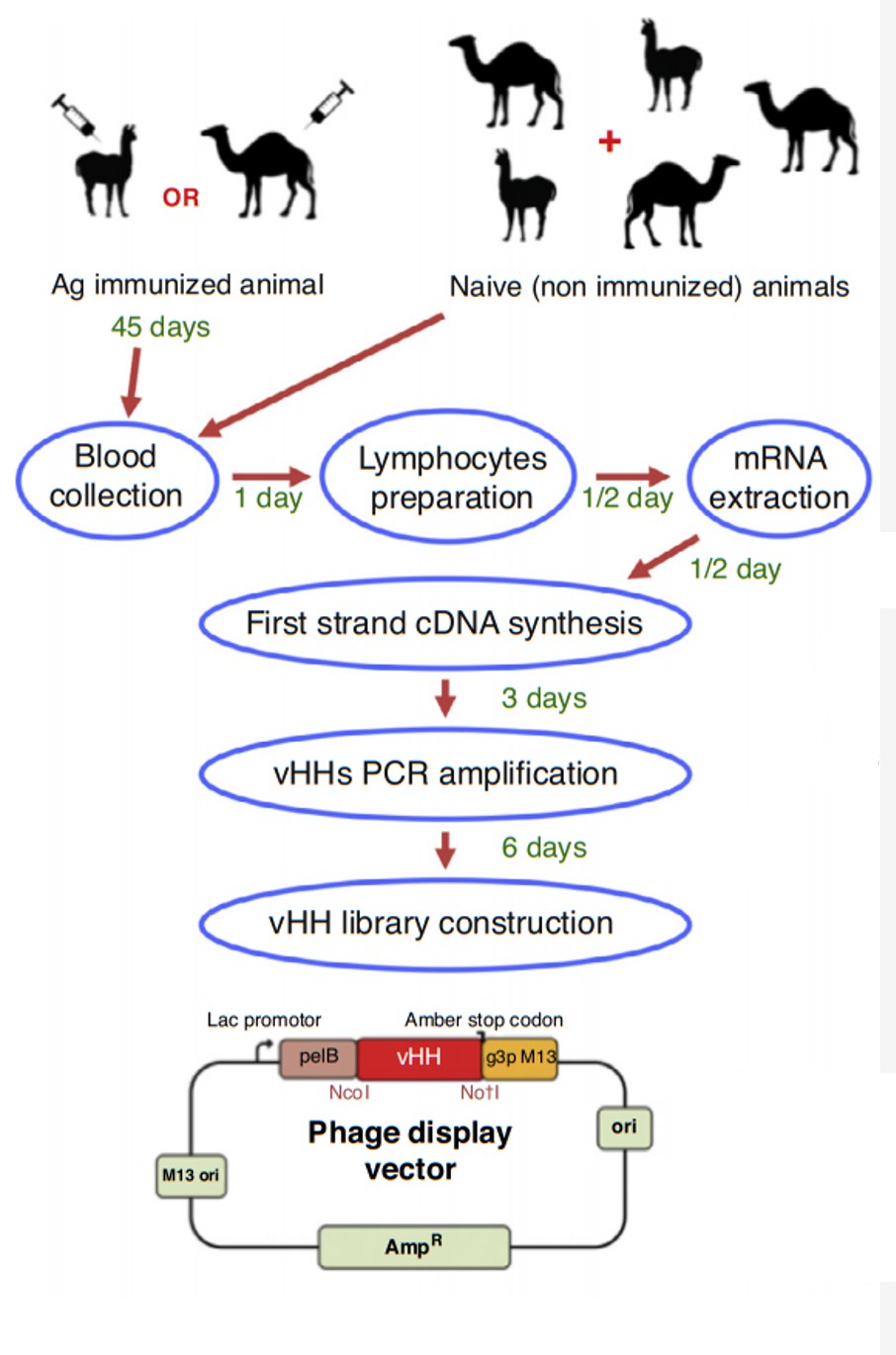
After the animals are injected with antigen, blood is collected for titration to monitor the immune response. Pre-immune serum will be tested with antiserum as a negative control.
We fixed the targets directly on the surface of the well plate for solid phase screening. After three rounds of screening, good enrichment effects were obtained, and there was also a significant difference compared with the control group.
About Target-1, we got the positive clones and then finished DNA sequencing. Finally, 7 unique clones were obtained. We then performed a validation by QC soluble ELISA, and the results showed that all clones could recognize the target.
The article presents a comprehensive study on the development of anti-membrane type 1-matrix metalloproteinase (MT1-MMP) sdAbs for positron emission tomography (PET) imaging in triple-negative breast cancer (TNBC), underscoring the aggressive nature of
TNBC and the significance of identifying biomarkers for improved patient outcomes. The article describes how a llama-derived sdAb library was generated and screened against the catalytic domain of MT1-MMP, resulting in the discovery of particular
sdAbs. Following a comprehensive analysis, two of these sdAbs were tagged with gallium-68 (68Ga) and tested in a TNBC mouse model. The immunoPET imaging exhibited precise in vivo tumor targeting with high signal-to-background ratios,
with (68Ga) Ga-NOTA-3CMP75 exhibiting more tumor uptake than (68Ga) Ga-NOTA-3TPA14. The imaging data was shown to be perfectly correlated with immunohistochemical staining results, demonstrating the sdAbs' selectivity for MT1-MMP.
The results show that anti-membrane protein sdAbs have great specificity against MT1-MMP, making them intriguing candidates for PET imaging in TNBC and warranting further exploration as diagnostic tools.
1. What is the significance of targeting membrane proteins with VHH antibodies?
Including various proteins with differences structurally and functionally, membrane protein is associated with a variety of important physiological and homeostatic processes, such as signal transductions, molecules and ions transfer, enzymatical actions,
and interactions between cells. Targeting these proteins with VHH is significant for fundamental research in studying cellular functions and in seeking potential therapeutic targets for membrane-related diseases.
2. What are the benefits of targeting membrane proteins with VHH antibodies?
Membrane proteins serve important roles in different physiological processes and are frequently implicated in illnesses, and therefore targeting them with VHH antibodies is significant. Because of their tiny size and strong affinity, VHH antibodies can
better access and bind to membrane proteins, making them valuable research tools and possible therapeutic agents.
3. What are the advantages of VHH antibodies in recognizing conformational epitopes of membrane proteins?
Compared to conventional antibodies, VHH antibodies have a unique ability to recognize and stabilize specific conformational states of membrane proteins. Their single-domain nature allows for a higher degree of flexibility in binding interactions, which
is particularly useful for studying dynamic conformational changes in membrane proteins.
4. What role do VHH antibodies play in the structural determination of membrane proteins?
VHH antibodies are useful in the study of membrane proteins using crystallography and cryo-electron microscopy through techniques such as X-ray crystallography and cryo-electron microscopy. They can aid in high-resolution structural research by facilitating
crystallization or stabilizing specific conformational states due to their high affinity binding to membrane proteins.
5. How can VHH antibodies enhance the specificity of membrane protein detection and quantification?
VHH antibodies have great specificity and affinity against particular membrane protein targets, allowing them to discriminate between closely similar membrane proteins and detect minute levels. This high specificity and strong affinity are useful in experiments
like Western blotting, immunoprecipitation, and flow cytometry, thereby enhancing the reliability of membrane protein detection and quantification.
6. What are the benefits of VHH antibodies in therapeutic and diagnostic applications involving membrane proteins?
VHH antibodies' small size and excellent specificity allow for greater tissue penetration and targeting. They can be designed to deliver therapeutic payloads or act as diagnostic agents with better pharmacokinetics than traditional antibodies. Leveraging
their high specificity and affinity to identify disease-related markers on membrane proteins improves diagnostic accuracy and efficiency.
7. In what ways can VHH antibodies be used to modulate the function of membrane proteins?
VHH antibodies can function as allosteric modulators, inhibitors, or agonists of membrane proteins. Their precise binding capabilities enable researchers to control membrane protein activity, investigate receptor-ligand interactions, and examine signal
transduction pathways with greater accuracy.
8. What is the impact of VHH antibodies on the advancement of membrane protein research techniques?
VHH antibodies have revolutionized numerous research approaches, including single-particle cryo-electron microscopy (cryo-EM). They can be utilized as fiducial markers or to stabilize proteins in certain states, thereby dramatically improving the resolution
and quality of the resulting structures.
9. How do VHH antibodies contribute to the overall understanding of membrane protein biology?
VHH antibodies are unique because they allow for extensive research of membrane protein structure, function, and interactions. This facilitates our experiments in membrane-related studies and improves our understanding of membrane protein biology, which
is essential for the development of new medications and therapies for membrane-associated disorders.


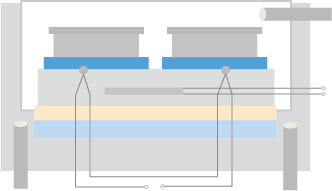
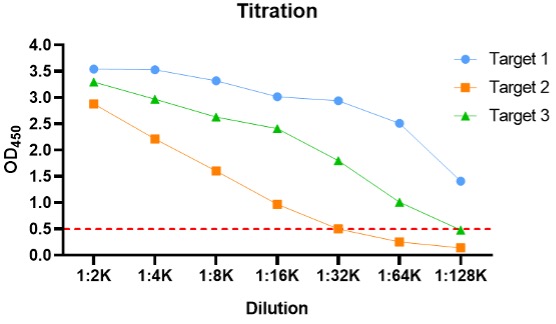 Figure 1. 2nd titration results.
Figure 1. 2nd titration results.
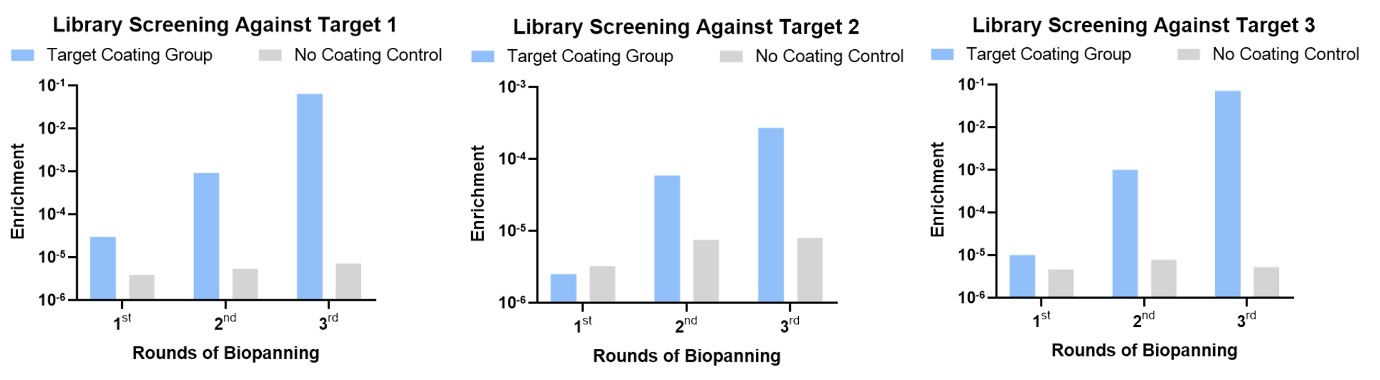 Figure 2. Process monitoring of library screening stage.
Figure 2. Process monitoring of library screening stage.
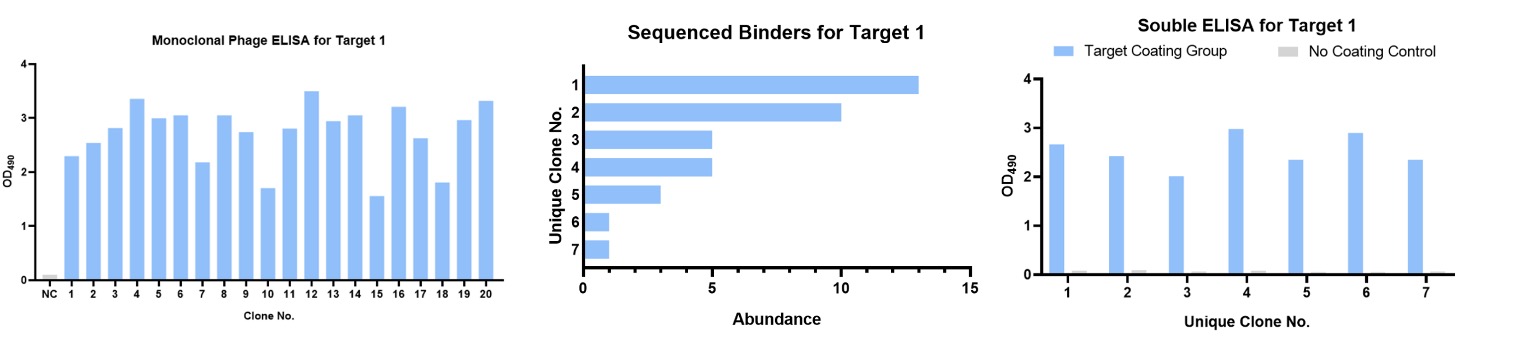 Figure 3. Summary of DNA sequencing and QC soluble ELISA for Target-1.
Figure 3. Summary of DNA sequencing and QC soluble ELISA for Target-1.
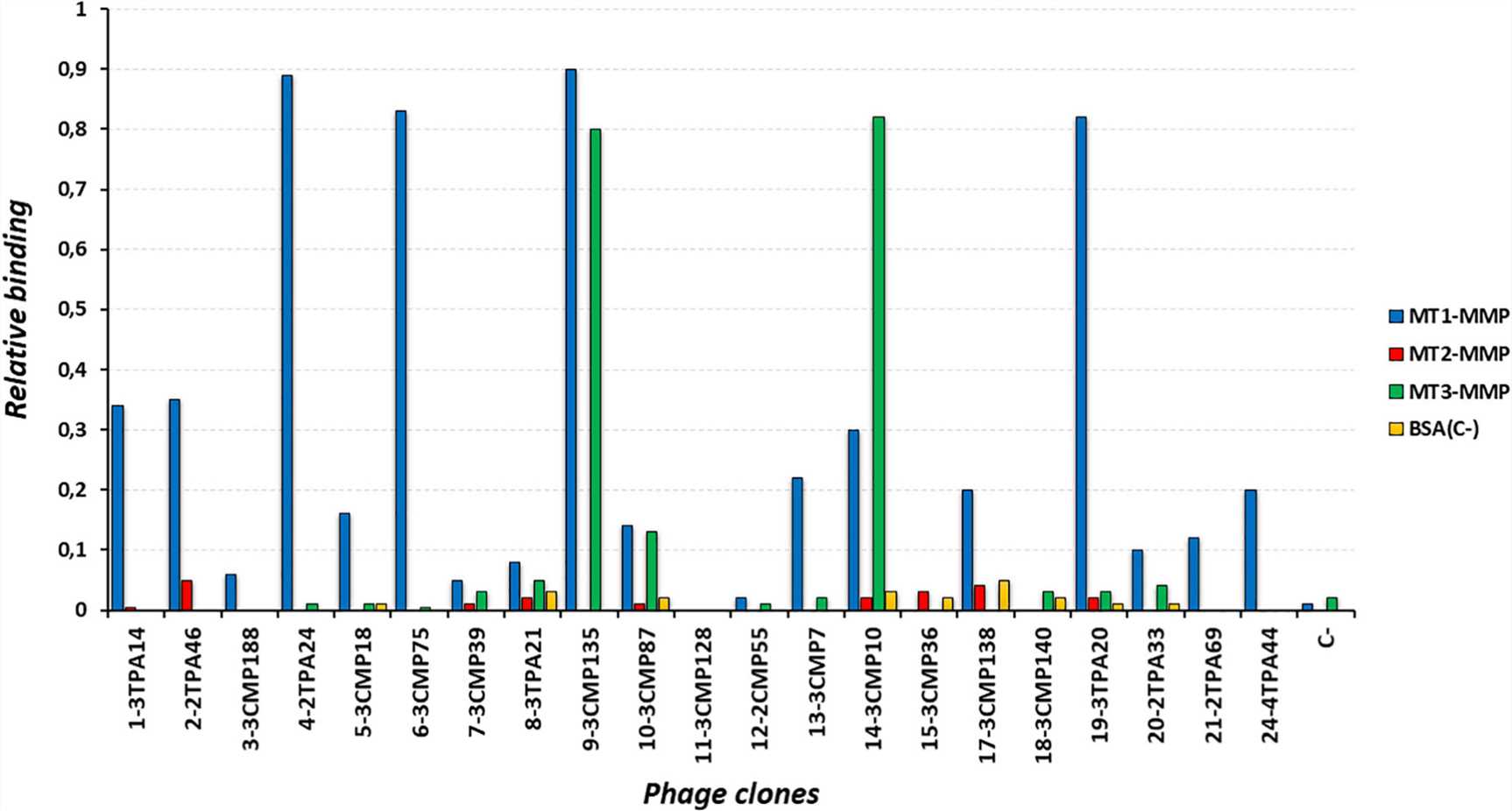 Fig. 1 SdAbs from Different Clones Demonstrate Specific Reactivity against MT1-MMP.1
Fig. 1 SdAbs from Different Clones Demonstrate Specific Reactivity against MT1-MMP.1
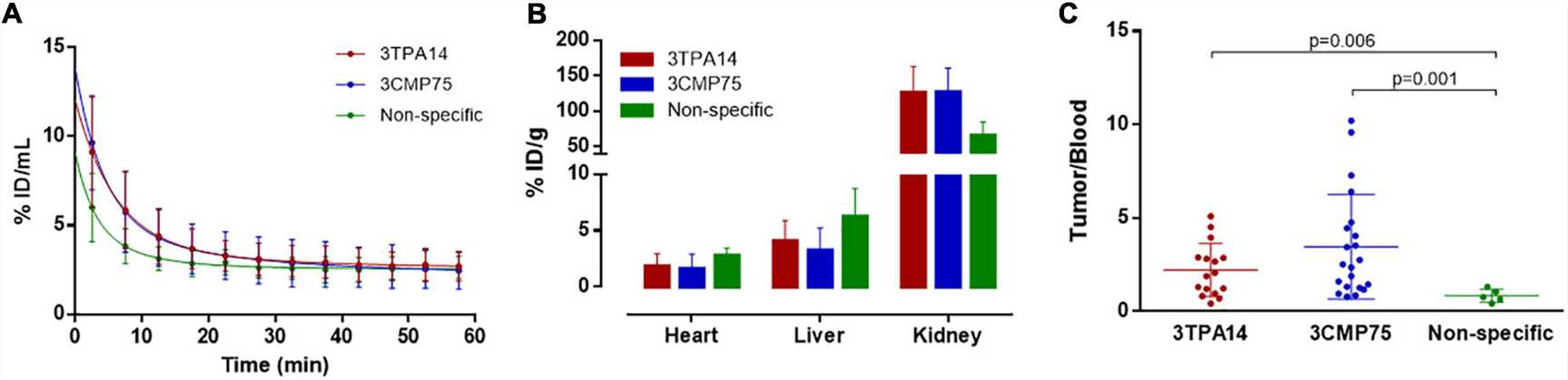 Fig. 2 Biodistribution and Pharmacokinetics of [68Ga] Ga-NOTA-sdAbs.1
Fig. 2 Biodistribution and Pharmacokinetics of [68Ga] Ga-NOTA-sdAbs.1