Endotoxin Detection Service
The endotoxin molecules consist of three parts: O-specific polysaccharide chain, core oligosaccharide and lipid A. Lipid A is the active moiety of the lipopolysaccharides (LPS) biochemical structure, which is responsible for LPS molecules' biological function, specificity, and affinity to the relative proteins. The O-specific polysaccharide chain can help bacteria evade the immune system.
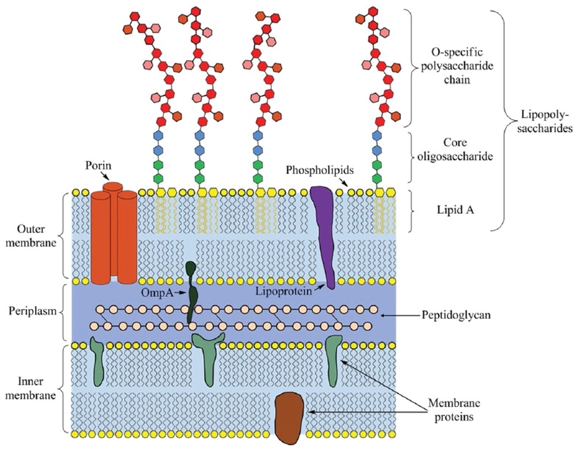 Fig.1 Illustration of the lipopolysaccharide biochemical structure. (Su, 2015)
Fig.1 Illustration of the lipopolysaccharide biochemical structure. (Su, 2015)
Our Services
The detection of endotoxin is an important part of ensuring the safety of sterilized products, especially in the fields of biological products, medical devices, parenteral drugs, food and water security, and so on. Even small amounts of endotoxin can influence health. Creative Biolabs has been committed to the development of endotoxin testing for many years. We employ sensitive, accurate and rapid methods for endotoxin detection. We customize the most suitable assay for each project and use an assay or combination of several following assays to meet your requirement.
Assay Content
-
Biosensor Technique
The biosensor technique is a kind of modern endotoxin detection method which will eventually replace the gold standard of RPT and LAL tests.
-
Recombinant Factor C Assay
The rFC assay, an endotoxin sensitive synthetic protein, is cloned from factor C DNA to use as an alternative in vitro LAL test.
-
Monocyte Activation Test
The monocyte activation test allows the detection of the full range of pyrogens including endotoxins and non-endotoxin pyrogens to use as a human alternative in vitro RPT.
Apart from the above assays, endotoxin detection methods also include the rabbit pyrogen test and bovine whole blood assay, although rarely used.
The rabbit pyrogen test was the first method widely approved for LPS detection in pharmaceuticals. This method was developed based on any rise in body temperature and symptoms of fever after injecting rabbits with the test liquid. However, the rabbit pyrogen test has obvious defects, such as being time-consuming and expensive. Moreover, its application is also limited to products that do not cause adverse effects in test animals. As a result, it has been gradually replaced by the LAL test in practical use. At present, this method is only used as a supplement of the LAL test to analyze pyrogenic materials in the earlier development phase of parenteral devices.
The mechanism of this method is to take the whole blood from the animal and introduce it to a solution containing the pharmaceutical being tested. Similar to humans, white blood cells in the bovine whole blood produce the cytokine Prostaglandin E2 (PGE2) is the response to endotoxin and the production of PGE2 is directly proportional to an increase in endotoxin concentration. Likewise, this assay has limitations. It is difficult to amass in vast quantities and religious practices in some countries.
Creative Biolabs is a world-famous service provider for bacteria endotoxin testing. We continue to innovate our endotoxin detection technologies to achieve even greater results. You could purchase the corresponding endotoxin detection products or send samples to seek our one-stop endotoxin detection service. For more detailed information on our endotoxin detection service, please feel free to contact us or directly send us an inquiry.
References
-
Su, W.; et al. Methods of endotoxin detection. Journal of Laboratory Automation. 2015, 20(4): 354-364.
-
Dullah, E.C.; et al. Current trends in endotoxin detection and analysis of endotoxin-protein interactions. Crit Rev Biotechnol. 2017, 37(2): 251-261.
For Research Use Only | Not For Clinical Use


 Fig.1 Illustration of the lipopolysaccharide biochemical structure. (Su, 2015)
Fig.1 Illustration of the lipopolysaccharide biochemical structure. (Su, 2015)
 Download our brochure
Download our brochure