In a new preclinical study, researchers from Weill Cornell Medicine found that genetically engineered T cells successfully targeted specific cancer cells that may lead to relapse in acute myeloid leukemia (AML), and demonstrated the effectiveness of this approach in an animal model of AML. The new cell therapy, which is currently in Phase 1 clinical trials, may eventually help AML patients remain cancer-free. Related research results were recently published in Nature Communications with the title “Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia”.
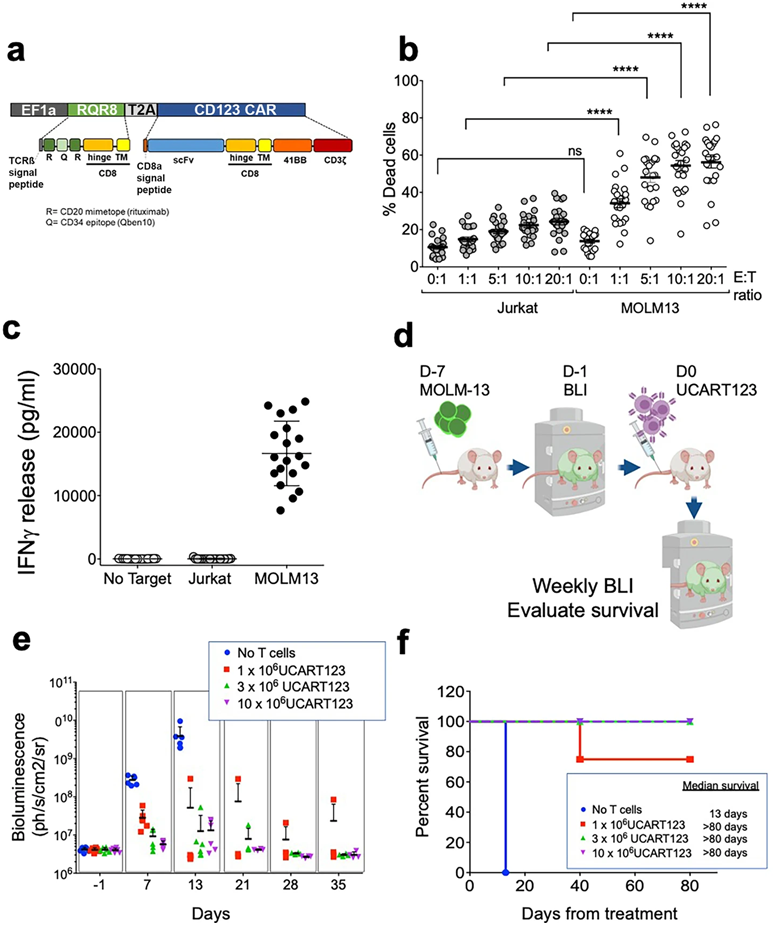
In this new study, the authors got T cells to produce chimeric antigen receptors (CARs) that allow the T cells to recognize specific markers on the surface of cancer cells. In the case of this study, the CAR bound to the CD123 molecule on the surface of leukemia stem cells, enabling T cells expressing CAR-T cells to seek out and attack the leukemia stem cells.
Dr. Monica L. Guzman, co-corresponding author of the paper and associate professor of medical pharmacology in the Division of Hematology and Medical Oncology at Weill Cornell Medical College, and her lab have been working to design mouse models to test new anti-leukemia therapies that target CD123.
Although there are effective therapies to treat AML, the disease eventually relapses in most patients even after they achieve complete remission. Dr. Guzman and her colleagues hope to rid AML patients of any remaining leukemia stem cells by genetically modifying T cells to express CAR targeting CD123. CAR-T cells are an attractive anti-cancer therapy because they can be grown in large numbers in the laboratory.
Dr. Guzman introduced that the CAR-T cells that target CD123 used in this study, called UCART123 cells, have several important features. Being derived from healthy donors, they target a leukemia stem cell marker and are made into off-the-shelf cell products ready for patients anytime. They are specifically designed to minimize toxicity and, in the case of overproliferation, they can be eliminated with a drug called rituximab.
When the authors tested UCART123 cells in a mouse model of AML, they found that the therapy effectively eliminated the leukemic cells and prolonged survival. They also devised an ultrasensitive monitoring strategy to detect any residual cancer cells and to assess the persistence of UCART123 cells. Finally, they confirmed that UCART123 cells were specific for leukemia cells and minimally toxic to normal blood cells in mice.
This preclinical result brings about a phase 1 clinical trial of UCART123 in patients with relapsed/refractory AML at several sites in the United States, including New York-Presbyterian Hospital/Weill Cornell Medical Center.
Dr. Gail Roboz, director of the Clinical and Translational Leukemia Program at Weill Cornell Medical Center and an oncologist at New York-Presbyterian Hospital/Weill Cornell Medical Center, said these preclinical findings demonstrated that UCART123 cells are highly selective and specific in targeting AML, and anticipated that the technology developed in Dr. Guzman’s laboratory will help monitor AML patients treated with UCART123 and optimize their likelihood of success.
Reference
1. Sugita, Mayumi, et al. “Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia.” Nature communications 13.1 (2022): 2227.