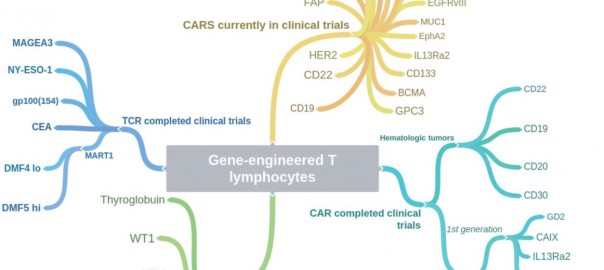
This is a review article published on Cell Research (2017) 27: 38-58 by Laura A Johnson and Carl H June. Dr. Carl H. June is the director of translational research at the Abramson Cancer CenterRead More…
The Latest Developments in CAR-T Research
CAR-T, Chimeric Antigen Receptor T Cell Immunotherapy, is a new cell therapy that has been developed for many years but has only been improved in recent years. Considered as one of the most promisingRead More…
Latest Research Results on CAR-T Therapy reveal a possible reason of CAR-T neurotoxicity
There is no doubt that this year marks the beginning of CAR-T therapy owing to the advent of two CAR-T therapies and the changes that these therapies have brought to the lives ofRead More…
FDA approval brings first gene therapy to the United States…
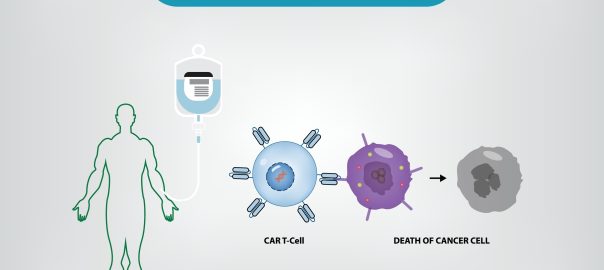
“First CAR T-cell Therapy – Kymriah created from Novartis was approved by the FDA in August 2017. Two months later, Yescarta developed by Kite Pharma, Inc) is the second gene therapy approvedRead More…
Actemra/RoActemra® Is Approved by FDA for the Treatment of Cytokine Release Syndrome Caused by CAR-T Therapy
Actemra/RoActemra® is the first FDA approved drug for treatment of serious or fatal cytokine release syndrome (CRS) caused by CAR-T therapy; CAR-T cell therapy is an immunotherapy used to treat certain typesRead More…
Chinese Scientists Discovered Strong Oncolytic Virus Synergist
On August 24th, a reporter was informed from Prof. Guangmei Yan research group at Zhongshan University that this group of researchers identified and gained oncolytic virus M1 synergist with accurate treatment of biomarkers. TheyRead More…
CAR-T for Autoimmune Disease and Blastoma Therapy
CAR-T for Autoimmune Disease An autoimmune disease is a pathological condition resulting from an abnormal immune response to tissues that can exist in the body, affecting up to 50 million Americans in termsRead More…
The Application of CAR-T in Disease Treatment
Chimeric antigen receptor (CAR) modified T cells (CAR-T) as a new field of adoptive immune cell therapy has become a hot spot in recent research. In 1989, Israeli scholar Gross et al.Read More…
Carl June Published His Latest Paper: The Results of CAR-T Therapy Human Trials on the Treatment of Solid Tumors Shows Two Hurdles Still Need to Be Overcome!
In this month, FDA oncologic Drugs Advisory Committee agreed to the approval of Novartis’s CAR-T CTL019 therapy on a vote of 10:0 for the treatment of relapsed or refractory children and youngRead More…
Is Bio-distribution Study Necessary for CAR-T Therapy?
Biological therapy includes a variety of models, such as immunotherapy, gene therapy, regulation of angiogenesis therapy, small molecule targeting drugs and stem cells and tissue engineering regenerative medicine. Among them, cancer immunotherapyRead More…