In a recent study conducted by researchers from institutions including the MD Anderson Cancer Center at the University of Texas, USA, it has been discovered that the loss of metabolic capacity in natural killer cells expressing chimeric antigen receptors (CAR-NK cells) is a critical mechanism for treatment resistance. CAR-NK cells infused into the body gradually lose their ability to compete for nutrients with tumor cells, leading to tumor recurrence. The findings of this study were published in the Science Advances journal on July 28, 2023, under the title “Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering.”
This research highlights that by genetically modifying CAR-NK cells to express interleukin-15 (IL-15), their metabolic capabilities can be enhanced, resulting in a more sustained anti-tumor response. Furthermore, compared to a single infusion, double infusions of CAR-NK cells expressing IL-15 can improve survival rates.
The lead authors of the paper are Dr. Ken Chen, a professor of Bioinformatics and Computational Biology at the MD Anderson Cancer Center at the University of Texas, and Dr. Katayoun Rezvani, a professor of Stem Cell Transplantation and Cellular Therapy.
According to Dr. Chen, “Understanding this mechanism of treatment resistance is crucial for conducting more targeted research to identify strategies that can mitigate or circumvent this resistance mechanism and enhance the effectiveness of CAR-NK cell therapy.”
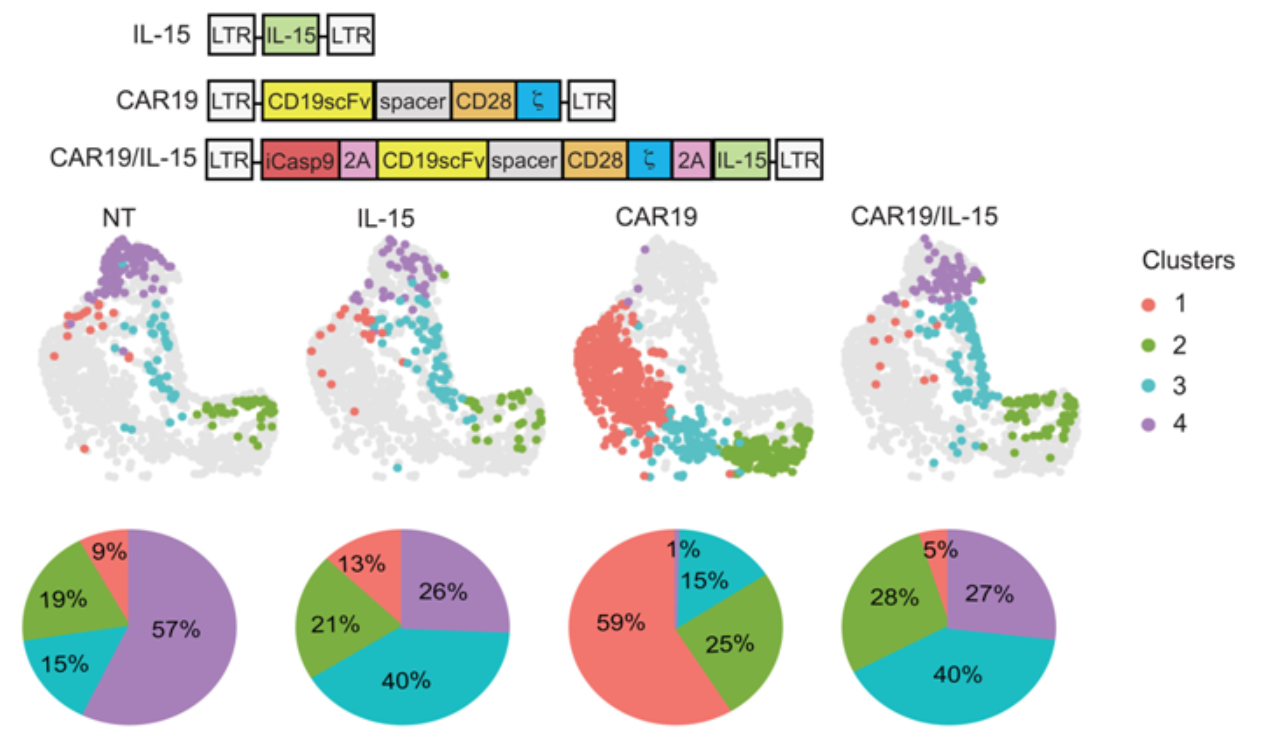
As part of preclinical research, the authors genetically modified NK cells targeting the CD19 tumor antigen with CAR (CAR19). They utilized a lymphoma laboratory model to evaluate the NK cells expressing CAR19 alone, those expressing IL-15 alone, or those expressing both CAR19 and IL-15, in order to study the activation of NK cells by IL-15 and its capacity to enhance their efficacy and persistence.
The authors observed that the activation, function, and metabolic characteristics of NK cell populations vary, correlating with different stages of in vivo evolution and tumor control. Interaction with metabolically active tumors can lead to NK cells losing their metabolic capacity, and the expression of IL-15 can partially overcome this metabolic loss.
To overcome this treatment resistance, mice received a second infusion of CAR19/IL-15 NK cells 14 days after the first infusion. This second infusion increased the number of functional NK cells, resulting in the long-term eradication of tumors. In a recent clinical trial, samples from two lymphoma patients received CAR19/IL-15 NK cell therapy, confirming the relevance of these preclinical research findings.
Dr. Rezvani stated, “Our data suggest that successful therapy using CAR-NK cells may require multiple infusions to generate a potent NK cell population capable of eliciting a strong anti-tumor response, especially for challenging-to-treat and metabolically active tumors.”
The increase in functional NK cells after the second infusion provides a clinically feasible strategy for enhancing the efficacy of CAR-NK cell therapy, deserving further investigation. The authors will continue to validate their findings in more tumor models and study treatment resistance mechanisms in an upcoming clinical trial using CAR-NK cells targeting CD70 for solid tumor therapy.
Reference
1. Li, Li, et al. “Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering.” Science Advances 9.30 (2023): eadd6997.