Immunotherapy strategies aim to utilize the patient’s own immune system cells to eliminate tumor cells. CAR-T cell therapy is an effective treatment for human blood cancers. In France, approximately 35,000 people are affected by blood cancers each year, with around 1.24 million new cases globally. Scientists from institutions such as the Pasteur Institute have discovered through their research on CD4 T cells, a type of immune cell involved in the treatment process, that CD4 T cells can neutralize tumor cells from a distance by producing interferon-gamma (IFN-γ). The research titled “Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4+ CAR T-cell antitumor activity” has been published in the international journal, Nature Cancer.
This research brings hope to patients who have shown incomplete responses to CAR-T cell therapy, as well as to cancer patients who are sensitive to IFN-γ. CAR-T cell therapy is a specialized form of immunotherapy that has shown significant effectiveness in treating certain forms of leukemia or lymphoma. However, some patients receiving this therapy experience disease relapse as the tumor cells in their bodies evade the treatment. In this study, researchers began to elucidate how this therapy works to generate more effective treatment responses in patients.
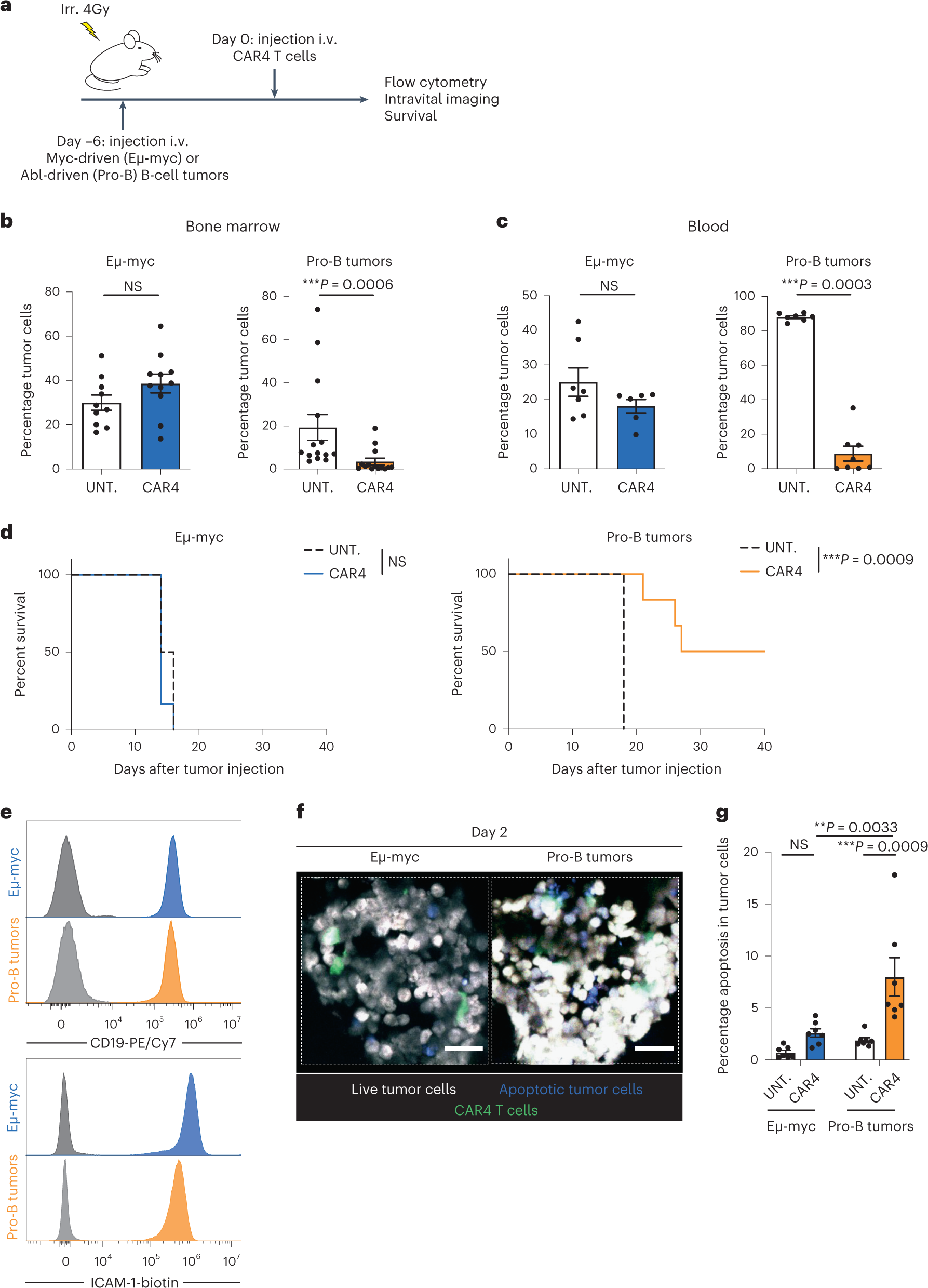
The principle of CAR-T cell therapy involves isolating the patient’s own T cells, genetically modifying them to specifically target tumor cells, proliferating them, and then infusing them back into the patient’s body. This army of cytotoxic CAR-T cells includes both CD4 T cells and CD8 T cells, and the composition of CAR-T cells varies among patients. Although researchers know that CD8 cytotoxic T cells require direct contact with tumor cells to destroy them, the molecular mechanisms behind the actions of CD4 T cells remain largely unclear.
Therefore, researchers studied these CD4 CAR-T cells in greater detail and revealed a fascinating characteristic: these cells can remotely eliminate tumor cells by secreting IFN-γ, a molecule involved in immune responses. Dr. Philippe Bousso explained that this destructive mechanism is highly effective for certain types of cancers that are sensitive to IFN-γ. The researchers also observed that patients who carry a large number of CD4 T cells and produce high levels of IFN-γ tend to respond better to the therapy.
To uncover the novel mechanisms of action of these remote cytotoxic cells, researchers began exploring clinical models to analyze specific details, particularly using in vivo imaging techniques, followed by examining the relevance of the results to patient samples. This research discovery may provide new avenues for adjusting therapies to prevent tumor cells from evading CAR-T cell therapy, offering new treatment prospects for patients, and helping develop a more personalized approach, utilizing a substantial number of CD4 CAR-T cells to activate IFN-γ based on tumor cell sensitivity.
By gaining a better understanding of the molecular mechanisms underlying the actions of CD4 cytotoxic T cells, researchers may be able to extend the scope of this treatment to other solid tumors sensitive to IFN-γ. Clinical research data can validate these findings in other cohorts. In conclusion, the researchers propose in this study that the sensitivity of tumor cells to the pro-apoptotic activity of IFN-γ is a major determinant of the efficacy of CD4+ CAR-T cell therapy, thereby guiding the utilization of CD4+ T cells during immunotherapy.
Reference
1. Boulch, Morgane, et al. “Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4+ CAR T-cell antitumor activity.” Nature Cancer (2023): 1-16.