Bordet, the winner of the 1919 Nobel Prize in Physiology or Medicine, found that when serum was heated to 56°C, it lost its ability to kill bacteria, even though the antibodies in the serum remained intact and could still interact with the antigen. From this observation, Bordet deduced that serum must contain some hitherto undiscovered component that was heat-sensitive, very fragile, and extremely small but could be supplemented by antibodies that would enable it to interact with bacteria and thus destroy them. He named this component “defensin,” which was later renamed “complement.”
Complement is now known to be a multimolecular system consisting of over 30 soluble proteins, membrane-bound proteins, and complement receptors, hence its name “complement system.”
The complement system has three pathways of activation:
- Classic Pathway: It begins with C1q-C1r2-C1s2, and its primary activator is the antigen-antibody complex.
- Alternative Pathway: It starts with C3 and does not depend on any antibodies.
- Lectin Pathway (MBL Pathway): The MBL Pathway is recognized by the Mannan Binding Lectin (MBL) glycosyl group.
- These three pathways share a common terminal pathway, namely the formation of membrane attack complexes and their lytic cellular effects.
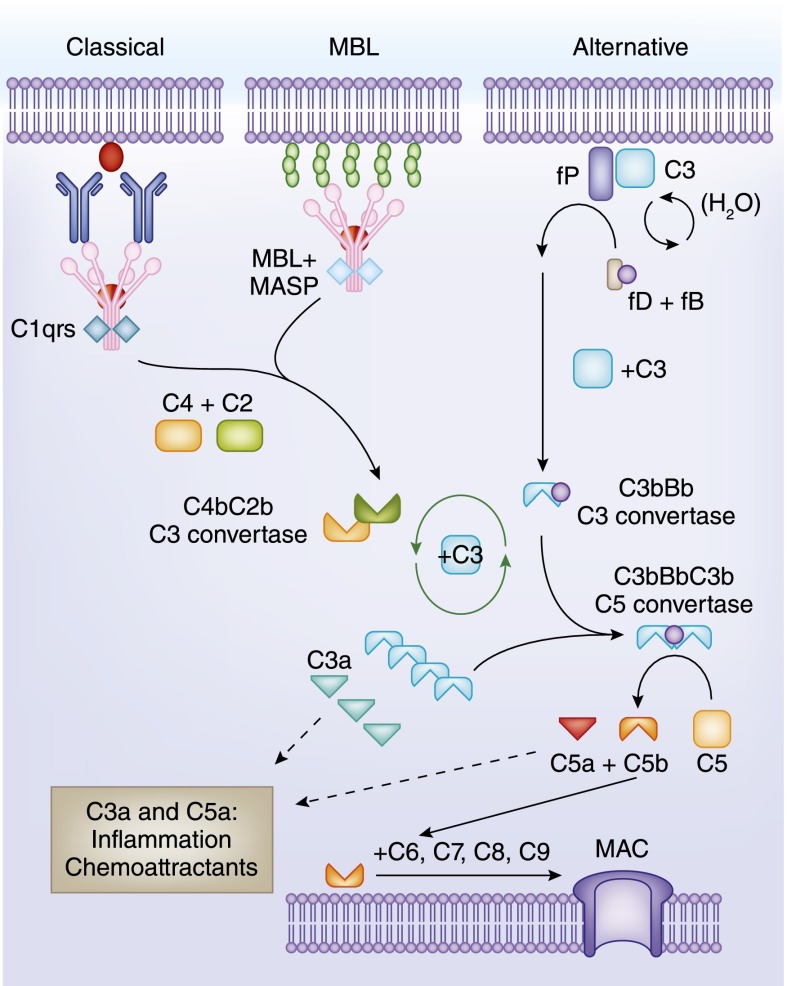
Fig. 1 Overview of the complement cascade. (Mathern D R and Heeger P S, 2015)
The Importance of Complement Test in Preclinical Drug Development
A class of therapeutic drugs that do not directly target complement factors but whose pharmacological efficacy results may have an impact on the stability of the complement system. For example, the specific Fc effector fragment of a common antibody class of drugs is masked at the complement recognition site when it does not bind antigen, and when it binds antigen, the complement recognition site is captured by C1q and activates the complement system. In normal animals, it is possible to cause a type III hypersensitivity reaction if the drug causes abnormal activation of complement. The detection of complement can be an important indicator for the interpretation of pharmacological efficacy as well as toxic side effects.
In the other category, therapeutic agents directly target complement factors, and these areC Eculizumab, Raculizumab, Narsoplimab, Avacopan, etc., which are mainly complement inhibitors. These drugs are mainly used in the treatment of rare diseases and autoimmune disorders. The detection of complement can directly characterize whether the drug is working or not.
In addition, for preclinical animal experiments, when animals exhibit flushing, chills, fever, tachycardia, hypertension, dyspnea, nausea, vomiting, syncope, or even death, the complement index test can be used as a piece of evidence to infer the cause.
In addition to the changes in complement homeostasis caused by the drug itself, it has been reported in the literature that the excipients or delivery systems of some drugs may also pose a risk of acute immunotoxicity in the form of hypersensitivity reactions (HSR). Complement activation via the classical and alternative pathways produces C3a and C5a allergenic toxins, causing “complement activation-related pseudo-allergy” (CARPA). These drugs include radiographic contrast agents (RCM), liposomal drugs (Doxil, Ambisome, and DaunoXome), and micellar solvents containing amphiphilic lipids (e.g., Cremophor EL, a carrier for paclitaxel).
Also, in recent years, lipid nanoparticles (LNPs) have shown their utility as delivery systems in RNA vaccines and therapies. In turn, LNPs have the potential to cause CARPA. Therefore, the currently popular mRNA-LNP drugs, as well as LNP-CRISPR gene therapy drugs are more inclined to detect complement indicators.
Significance of the Assay for Different Fractions of Complement
This is because there are three pathways of complement: classical, alternative, and MBL, and each pathway has similarities and differences. Therefore, the choice of which complement component to detect needs to be carefully considered according to the study’s purpose.
CH50 Assay
- To observe changes in the total complement activity of the classical pathway, the CH50 assay can be chosen. It measures the amount of complement required for 50% erythrocyte lysis.
- Significance of the CH50 assay: Changes in total complement activity indicate the status of the terminal complement pathway in the sample by indirectly reflecting the concentration of TCC. It is important for diagnosing and monitoring disease efficacy.
- Three allergenic toxins are produced in the classical pathway of complement: C3a, C4a, and C5a, where C3a is produced during the activation of the classical, alternative, or MBL pathways. C3a has been shown to increase vascular permeability, be spasmogenic and chemotactic, and induce the release of pharmacologically active mediators from various cell types.
- C3a is a low molecular weight (~9kD) protein fragment consisting of 77 amino acids. It is short-lived and rapidly metabolized by the serum enzyme carboxypeptidase N into the more stable 76 amino acid form called C3a-desArg (C3a C-terminal dearginine form). The kit typically utilizes an antibody sandwich method (monoclonal antibody + C3a + antibody-HRP) to detect the total sum of all forms of C3a.
- Significance of C3a detection: To study the role or status of the complement activation pathway, it is essential to monitor C3a production in vivo or in vitro.
- Under normal conditions, activation of the classical, alternative, or MBL pathways results in the formation of a C5 convertase multimolecular enzyme capable of cleaving C5 into C5a and C5b. As the most potent complement allergenic toxin, C5a has many biological functions, including mast cell degranulation, the release of inflammatory mediators, chemotaxis, leukocyte sequestration, and cellular activation through binding to C5a receptor 2 (C5aR or CD88).
- Similarly to C3a, C5a is a low molecular weight (~9kD) protein fragment containing 74 amino acids. C5a is rapidly metabolized by the serum enzyme carboxypeptidase N into the more stable 73 amino acid form called C5a-desArg (C5a C-terminal dearginine form). Usually, the kit uses an antibody sandwich method (monoclonal antibody + C5a + antibody-HRP) to detect the sum of all forms of C5a.
- Significance of C5a detection: C5a is an important component of the terminal complement complex and plays multiple functional roles. To study the role or state of terminal complement pathway activation, the production of C5a needs to be monitored in vivo or in vitro.

Fig. 2 Alternative pathway activation process. (Noris M and Remuzzi G, 2013)
- Bb is a specific component of the alternative pathway. In the absence of specific antibodies, it provides the body with protection against microbial attack through the complement alternative pathway. An important reaction in the activation of the alternative pathway is the conversion of factor B (93Kd) to the active protein hydrolase C3bBb.
- Significance of the Bb assay: By assessing the Bb content of the test sample, it is possible to estimate the extent of alternative pathway utilization at that time point for the sampled specimen.
- The terminal complement complex (TCC, SC5b-9) is the result of a disparate pathway resulting from the activation of the complement system by the classical, alternative, or MBL pathways. The membrane attack complex (MAC), which is a type of TCC, is a stable complex responsible for causing irreversible damage to target cell membranes upon complement activation. C5b-9 complexes formed in the absence of target membranes bind to naturally occurring regulatory serum proteins, like S proteins, to form steady-state SC5b-9. General kit assays utilize monoclonal antibodies that bind to the C9 loop of TCC targeting SC5b-9 and are followed by the addition of monoclonal antibodies that bind to the SC5b-9 antigen in complex with HRP.
- Significance of the SC5b-9 assay: It indicates the status of the terminal complement pathway in a sample by measuring the concentration of the TCC membrane attack complex, which exhibits cytotoxicity when its concentration is abnormally elevated.
Stability Considerations in Complement Assays
The quantification of natural complement components and total lysis complement activity is usually performed with serum samples. In contrast, measurement of complement activation products produced in vivo requires plasma samples because rapid in vitro activation may occur in serum. Baseline concentrations of activation products in serum are significantly higher than those in plasma.
Factors affecting in vitro complement activation are time, temperature, and anticoagulants (EDTA, citrate, and heparin). Ethylenediaminetetraacetic acid (EDTA) effectively binds calcium and magnesium, thus blocking both the classical and alternative pathways of complement, and is more effective than citrate and heparin in inhibiting in vitro activation, especially of the terminal pathway. Therefore, EDTA plasma is often recommended for complement activation assays. In addition, studies have shown that the inhibition present in plasma is eliminated when the temperature is increased over time. Therefore, to avoid in vitro activation, it is most important that the sample be kept at a constant low temperature.
In summary, to ensure accurate detection of complement activation products, sample collection should adhere to the following principles: collect blood samples directly into tubes containing EDTA and promptly store them at 4°C. Plasma should be immediately separated and stored at 4°C and needs to be stored frozen at -70°C for a maximum of 8 hours, with no significant activation occurring in the frozen state for 3 years. It is important to avoid repeated freezing and thawing during the assay process.
References:
- Mathern D R and Heeger P S. Molecules great and small: the complement system. Clinical Journal of the American Society of Nephrology: CJASN, 2015, 10(9): 1636.
- Ricklin D, et al. Therapeutic targeting of the complement system. Nature reviews. Drug discovery, 2019.
- Noris M and Remuzzi G. Overview of complement activation and regulation. Seminars in nephrology. WB Saunders, 2013, 33(6): 479-492.
