Background Services Overview Protocols Related Products Published Data Q&A Resources
Complement activation can be assessed by measurement of complement activation products, which reflect the
stimulation of any of the activating pathways. Creative Biolabs is one of the world's largest
suppliers of complement testing, providing customers with high-quality complement activation product test
services from diagnostics to drug development.
Background
Introduction of Complement Activation Products Test
In general, total hemolytic activity and individual component tests are useful as first-level screening
techniques, but they are not sensitive enough to detect pathologically increased complement activation in
vivo. The complement activation products can reflect the actual activation state of the complement
system under pathological conditions because their increase occurs only when the complement enzymes are formed
and complement is activated. An additional advantage is that the activation pathway of complement can
be determined according to the activation products. For example, C4a and C4d are markers for the classical
pathway (CP) or lectin pathway activation (LP); Bb is a marker for alternative pathway
(AP) activation; C3a, iC3b, C5a and sC5b-9 (soluble C5b-9 bound to S protein) can be used to determine the
terminal pathway. Complement activation products include split fragments after enzymatic cleavages of
certain components, such as C4 (C4a and C4d), C3 (C3a and C3d), factor B (Bb), C5 (C5a). In addition, some
protein complexes, like Clrs-C1 inhibitor, the properdin-containing alternative pathway convertase C3bBbP, and
sC5b-9, are also complement activation products.
Specimen Preparation
Sample preparation is very important before test. Complement activation products are usually present in
only
trace amounts in vivo, but they are rapidly generated in vitro. Therefore, samples must be
collected and stored correctly to avoid in vitro activation. Normally, blood should be drawn
directly
into tubes containing EDTA with a final EDTA concentration of 10 mM at least. Alternatively, nafamostat mesilate
can
be used as an anticoagulant, whereas citrate and heparin do not sufficiently block complement activation. The
sample
should be cooled, and plasma should be prepared immediately and stored at -80C.
Complement Activation Products Test
Over the last two decades, highly specific monoclonal antibodies have been generated which recognize only neoepitopes exposed to conformational changes induced by complement activation. Monoclonal antibodies to neoepitopes exposed in the activation product but hidden in the native component enable specific quantification of complement activation products. This assay is typically based on sandwich techniques such as ELISA, which uses the anti-neoepitope antibody as a capture antibody to directly capture activation fragment without the interference of the nonactivated component. A number of activation products can be tested through this method, including C4a, C4bc (such as common epitope for C4b and C4c), C4d, C3a, C3bc (such as common epitope for iC3b, C3b and C3c), C3dg, Ba, Bb, СЗbBЬP, C5a and soluble terminal C5b-9 complex (TCC) sC5b-9 (Fig.1). Furthermore, many of the neoepitope-specific antibodies can also be used to detect in situ complement activation by applying immunohistochemistry.
Services Overview
Our Services of Complement Activation Products Test
Creative Biolabs has developed a variety of specific monoclonal antibodies that can be used for the testing of
complement activation products. With our expertise in the field of complement analysis, we provide our customers
with top-quality complement activation products test services through a range of exclusive technologies to
accelerate their research. Testing services for multiple activation products of complement are available
at Creative Biolabs, including:
We will work with you to make sure you get the right services in the most economical manner, including:
-
The best available diagnostic test
-
Expert interpretation of all test results
-
Fast and efficient service
-
The best prices with the best after-sale service
Our test can provide valuable information about the specific symptom, diagnosis, and treatment of diseases. If
you have any questions, please do not hesitate to contact us.
Protocols
Related Products
Published Data
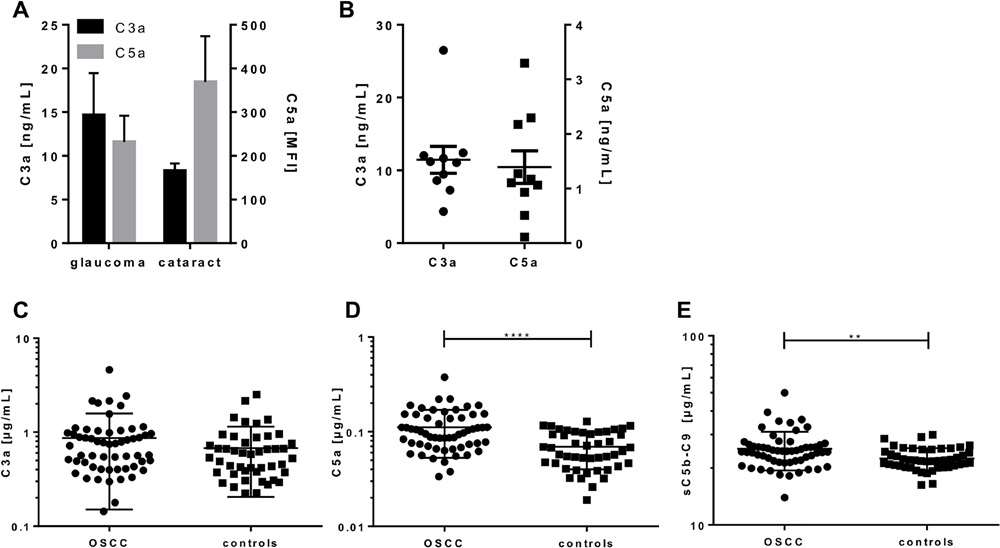 Fig.1 Quantification of complement activation markers in aqueous humor, tears, and serum from OSCC patients and healthy controls.1
Fig.1 Quantification of complement activation markers in aqueous humor, tears, and serum from OSCC patients and healthy controls.1
Oral squamous cell carcinoma (OSCC) represents a common cancerous condition affecting the oral region. Researchers sought to evaluate complement system activation in OSCC patients as a potential biomarker for disease progression. To this end, they developed a novel complement activation array, incorporating characterized antibodies that target specific complement epitopes, including C3a, C5a, and sC5b-9, within a suspension bead array format. This multiplexed test facilitates the identification of C3a, C5a, and sC5b-9 from minimal volumes of human biological fluids. Elevated systemic sC5b-9 levels correlated with invasive tumor growth, while C3a levels were linked to local tumor spread, suggesting their utility in assessing OSCC progression.
Resources
Reference
-
Gallenkamp, Juliane, et al. "A novel multiplex detection array revealed systemic complement activation in oral squamous cell carcinoma." Oncotarget 9.3 (2017): 3001-3013. Distributed under Open Access license CC BY 4.0, without modification.
Questions & Answer
A: Complement activation is a component of the innate immune system that helps eliminate microbes and damaged cells by attacking the cytoplasmic membrane. The complement system is composed of many small proteins, many of which can be used as markers of complement activation. It is generally recommended to measure complement activation products such as C3a, C5a, TCC, Bb, iC3b, C4d, SC5b-9, CH50, C3 convertase, and C5 convertase to detect complement activation.
A: Complement activation products C3a, C4a and C5a are fragments produced by complement activation and these are important products produced by complement activation. Activation of all three pathways produces C3a. C4a is induced in the classical and lectin pathways. C5a is the result of terminal pathway activation.
A: Cell-bound complement activation products (CB-CAPs) or complement split products, refers to complement activation fragments, C4d, that are bound covalently to somatic cells, as a result of activation of the classical complement pathway. They appear potentially useful for the diagnosis of systemic lupus erythematosus as of 2015.
A: The complement activation products test can be conducted on a variety of samples, including blood, plasma, serum, tissue, or cells. The source of the sample may impact the preparation required, so it is important to clarify this point when arranging for testing.
A: The test is highly accurate, however, it should be noted that the results can be influenced by multiple factors such as the state of the sample, the specific methodology used for the test, and the precision of the measurement. As such, it's crucial to ensure that the sample is handled properly and the experiment is conducted under optimal conditions.
A: The specificity of the complement activation products test depends on the specific markers that are being measured. Some markers may be specific to certain pathways of complement activation, while others may be produced by multiple pathways. It's important to choose your markers carefully based on what information you are seeking to gain from the test.
A: Yes, the complement activation products test can be a powerful tool for evaluating the impact of therapeutics on complement activation. By comparing the levels of complement activation products before and after treatment, you can gain insights into the therapeutic's effect on the immune response.
A: Sample preparation protocols may vary based on the source of your sample (eg, blood, cells, tissue). Some samples may require specific preparation steps like purification, concentration, or dilution. We will provide detailed instructions on how to properly prepare your samples based on their source.
For Research Use Only.
Related Sections:

 Fig.1 Quantification of complement activation markers in aqueous humor, tears, and serum from OSCC patients and healthy controls.1
Fig.1 Quantification of complement activation markers in aqueous humor, tears, and serum from OSCC patients and healthy controls.1