The increasing number of clinical diseases involving pathological contributions of the complement system has prompted renewed interest in therapeutic options to modulate this host defense pathway. Based on these findings, the pharmaceutical industry has identified the complement system as a potential and promising target for the treatment of various diseases.
The complement system protects the host from the harmful microbial environment and maintains tissue and cellular integrity by eliminating altered or dead cells. As an important effector arm of innate immunity, it also plays a central role in the regulation of acquired immunity. Thus, complement activation may drive the pathology of hypertension through its effects on innate and acquired immune responses.
Researchers from the University Medical Center Hamburg-Eppendorf recently published a study in Br J Pharmacol entitled “Complement component C3 as a new target to lower albuminuria in hypertensive kidney disease.” The study found increased C3 expression in the kidneys of hypertensive mice and men. Gene and therapeutic C3 knockout improved proteinuria in the early stages of hypertension, but not arterial blood pressure, renal injury, or cardiac injury.
The effects of complement activation on immunity and tissue integrity may contribute to hypertension. The researchers investigated the expression of C3, a key protein in the complement cascade, in relation to hypertension.
C3 expression was increased in renal biopsy tissue and glomeruli from patients with hypertensive nephropathy. Renal single-cell RNA sequence data from normotensive and hypertensive patients confirmed that C3 was expressed in different cellular compartments of the kidney. Renal C3 expression was upregulated in angiotensin II (Ang II)-induced hypertension.
C3-/- mice showed a significant reduction in proteinuria in the early stages of hypertension. However, there was no difference in blood pressure, renal injury (histology, glomerular filtration rate, inflammation), or cardiac injury (fibrosis, body weight, gene expression) between C3-/- and wild-type mice after Ang II injection. In addition, in deoxycorticosterone (DOCA) salt hypertension, C3-deficient mice were found to have significantly reduced proteinuria during the first week of hypertension, but there were no significant differences in renal and cardiac injury.
Targeting GalNAc (N-acetylgalactosamine) small interfering RNA (siRNA) for C3 downregulation resulted in a 96% decrease in hepatic C3 and a reduction in early proteinuria, but it had no effect on blood pressure or visceral injury. Inhibition of complement C5 by siRNA did not impact proteinuria.
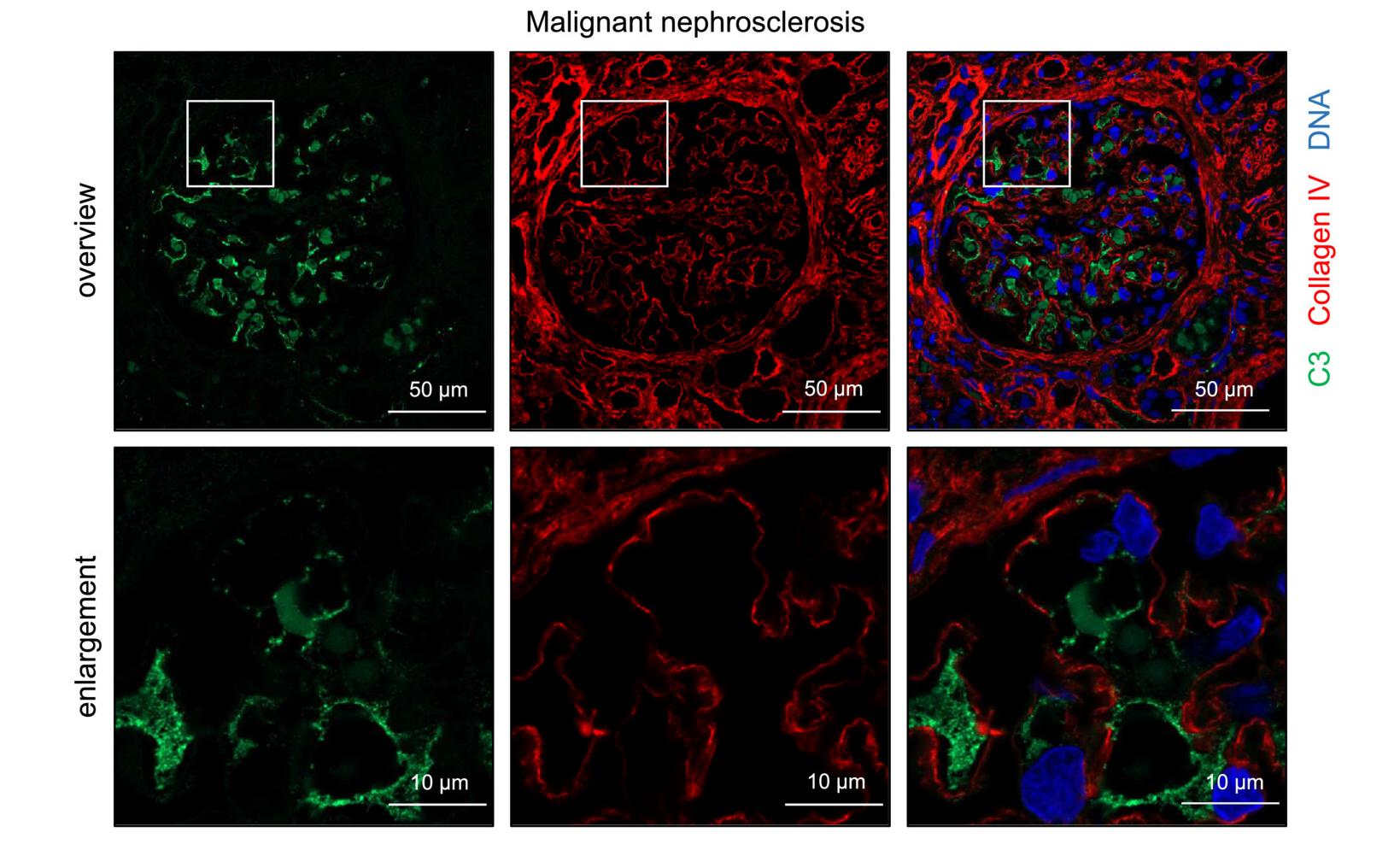
Fig. 1 Complement C3 expression in hypertensive nephropathy. (Marlies Bode et al., 2023)
The data from this study emphasize the complexity of the complement system in causing end-organ damage in hypertension. In both mouse and human hypertension, C3 displayed a significant induction and upregulation effect. In two different hypertension models and in two distinct pathways, C3 deficiency reduced proteinuria in the early stages of hypertension. However, this effect diminished with prolonged hypertension, and C3 deficiency did not lead to a reduction in structural renal and cardiac end-organ damage.
Further investigations are necessary to explore why C3 affects proteinuria early on in hypertensive patients rather than later. Many therapies targeting the complement system are currently at different stages of clinical development. The present study clearly indicates that C3 does not lower blood pressure and is not a therapeutic target for severe hypertensive end-organ damage as observed in the utilized model. Additional research is needed to determine whether C3 can serve as a marker of renal injury.
Current data suggest that targeted complementation may not be appropriate for the treatment of proteinuria in severe hypertensive nephropathy due to the lack of differences observed at longer time points. However, considering that proteinuria is the most crucial risk factor for the progression of chronic kidney disease, it is important to evaluate the mechanisms through which C3 inhibition reduces early proteinuria. Additionally, assessing whether targeted complementation could be beneficial in treating proteinuria in less severe models of arterial hypertension or other non-hypertensive models of kidney injury is also significant.
Reference:
1. Marlies Bode et al. Complement component C3 as a new target to lower albuminuria in hypertensive kidney disease. Br J Pharmacol. 2023 Apr 19.
