Exosomes, nanoscale extracellular vesicles enveloped by a lipid bilayer membrane and secreted by most eukaryotic cells, possess unique characteristics, including inherent stability, low immunogenicity, biocompatibility and excellent biological properties. Their membrane penetration capabilities enable them to function as highly efficient natural nanocarriers. Additionally, exosomes play a crucial role as a rich source of biomarkers in clinical diagnosis. A recent study conducted by researchers from the United States delved into the value of exosomes as a natural “liposome” in drug delivery and disease diagnosis. By synthesizing research data from the American Chemical Abstracts Service (CAS), they aimed to comprehend the clinical application field of exosomes, shedding light on valuable resources. This research was published in the journal ACS Nano on November 10 under the title “Exosomes—Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics.”

The current challenge in drug treatment lies in achieving effective targeted drug delivery. Numerous drug nanocarriers have been developed to enhance pharmacokinetics and pharmacodynamics while reducing toxicity and side effects. Many smart artificial drug delivery systems, such as various functionalized, stimulus-responsive, targeted lipid or polymer nanocarriers, have been developed to improve key characteristics of drug delivery systems, such as blood flow circulation time, biodistribution, cellular Interactions and drug loading and release. However, synthetic drug delivery systems still encounter setbacks such as non-specific drug targeting, carrier toxicity, immunogenicity, and suboptimal efficacy.
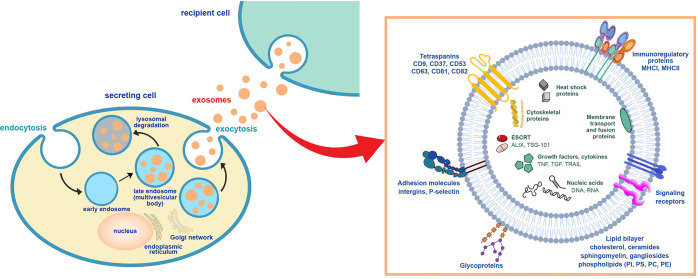
Lipid nanoparticles (LNPs), particularly liposomes, have emerged as promising carriers for protecting, transporting, and delivering various drugs and vaccines. Liposomes, early lipid nanoparticles, offer a flexible and resource-rich nanodrug delivery systems that significantly improves drug pharmacokinetics. Encapsulation within liposomes prevents drug dilution, and degradation, or inactivation in the blood. Lipid nanoparticle technology, along with other nanotechnology platforms, enhances the efficiency, selectivity, residence time, and biodistribution of traditional drug carriers while addressing their limitations. However, the clinical application of lipid nanocarriers faces challenges, such as low bioavailability, toxicity, easy removal from the blood, and stimulation of innate immune responses. Nearly two decades after the discovery of liposomes, scientists discovered that similar lipid vesicles naturally form in organisms, known as extracellular vesicles (EVs), which are secreted from cells and serve as normal physiological or certain pathological processes. Based on the origin and size of EVs, as well as the current understanding of their biogenesis, they can be divided into exosomes (approximately 30-150 nm in diameter), microvesicles (100 nm-1 μm) and apoptotic bodies (50 nm- 5μm). Exosomes, produced in endosomes within most eukaryotic cells, are subsequently released into the extracellular space through fusion with the cell biomembrane.
Exosomes play a pivotal role in facilitating efficient communication and signaling between cells, including the transport of bioactive molecules such as proteins, lipids, and nucleic acids across biological barriers. These unique physicochemical properties position exosomes as valuable tools in the field of drug delivery and diagnostics. Despite their recognized potential, current research on the physiology of exosomes remains insufficient. Upon the discovery of exosomes, their striking similarities to liposomes became evident, with exosomes representing more intricate versions derived from biological systems. Notwithstanding these parallels, exosomes exhibit distinct advantages that render them superior drug delivery vehicles. The lipid composition of exosomes, enriched with in non-lamellar-forming lipids, imparts a favorable curvature to their lipid bilayers, enhancing their capacity for drug delivery. Furthermore, exosome lipid bilayers possess a high degree of asymmetry, particularly facilitating their interaction with the plasma membrane, especially that of the target cell. Unlike liposomes, which are generally protein-free, exosomes contain a plethora of integral and peripheral membrane proteins, further enhancing their applicability in drug delivery. Therefore, exosomes are more suitable as potential drug carriers than lipid nanoparticles.
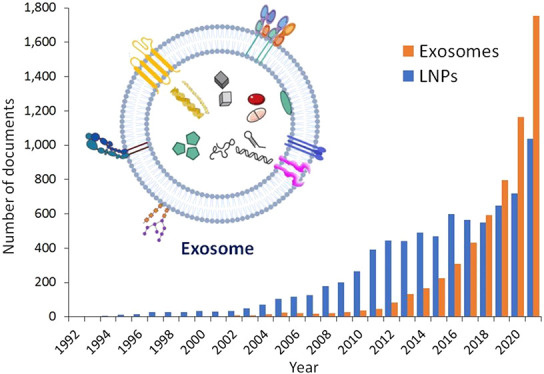
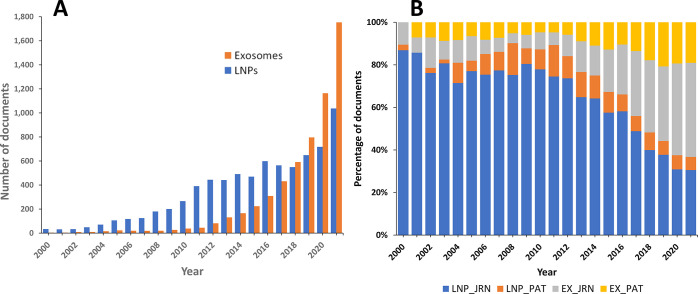
The results of a comprehensive search of the CAS Content Collection reveal a notable abundance of literature, encompassing patents and journal articles, related to the application of exosomes in drug delivery, surpassing that of lipid nanoparticles.
To improve the delivery efficiency of exosomes, scientists have gleaned valuable insights from the development of liposomes. Numerous technologies that have significantly improved liposome/lipid nanoparticle production and drug loading—such as sonication, extrusion, freeze-thaw cycles, and microfluidics—have been successfully applied to exosomes. Moreover, functional modifications that proved effective in enhancing liposome efficiency, including targeting specific cell receptors through surface-attached ligands and coating with biocompatible inert polymers like polyethylene glycol (PEGylation), have extended the circulating half-life of exosomes, rendering them invisible to phagocytes.
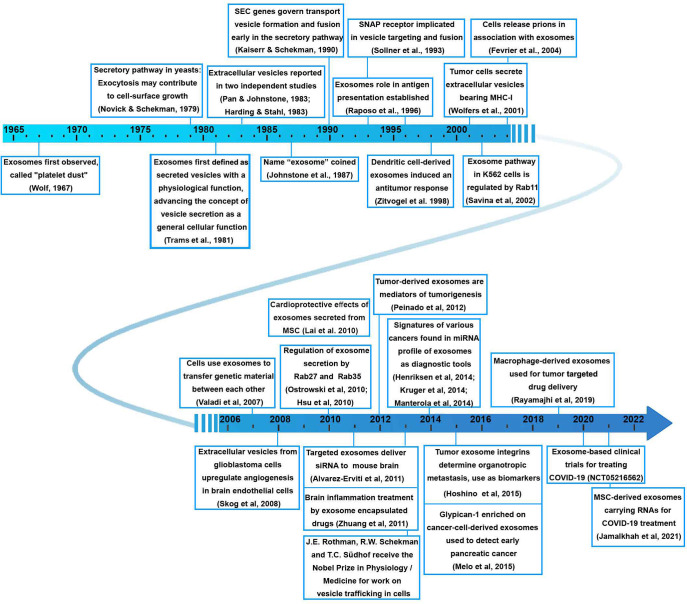
Exosomes, serving as a natural carrier platform, boast several inherent advantages. Originating from biological systems, their components are easily metabolized and excreted post-delivery. Exosomes generate minimal immune response and overcome traditional colloidal delivery system obstacles, particularly the blood-brain barrier (BBB), proving effective against challenging-to-deliver molecules such as proteins and RNA. A particularly promising clinical application of exosomes lies in diagnostics. Research indicates that exosomes contain proteins and nucleic acids associated with various diseases, including cancer, neurodegenerative, metabolic, infectious, and inflammatory conditions. Easily obtained from readily available body fluids like blood and urine, exosomes are thus ideal targets for diagnostic applications. This comprehensive review summarizes the evolving landscape of exosomes in drug delivery and diagnosis. Researchers meticulously curate insights from the CAS literature dataset, offering a panoramic view of recent advancements in the therapeutic and diagnostic applications of exosomes. The review discusses the composition and pathways of exosomes, from their biogenesis and secretion to uptake by recipient cells. It evaluates methods for exosome isolation and purification, explores their clinical applications in therapeutics and diagnostics, and scrutinizes their development pipelines, company discovery targets, and classifies diseases they aim to treat. The researchers aspire that this review contributes to the current knowledge of exosomes in medical applications, paving the way for addressing remaining challenges in the field.
Reference:
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes─Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano. 2022;16(11):17802-17846. doi:10.1021/acsnano.2c08774
Related Services:
Exosome Cargo Loading Services
Exosome Lipidomics & Metabolomics Services
