It is well known that tumor metastasis relies on microenvironmental support from remote organs. However, the mechanisms through which metabolism regulates the tumor metastasis microenvironment remain undetermined. Recently, researchers discovered that lung mesenchymal cells, rich in lipids, deliver lipids to tumor cells and NK cells via exosome-like vesicles. This process reshapes the pre-tumor microenvironment, promoting breast cancer lung metastasis. The relevant research published in the journal Cell Metabolism titled “Lipid-laden lung mesenchymal cells foster breast cancer metastasis via metabolic reprogramming of tumor cells and natural killer cells.”

Ninety percent of solid tumor-related deaths result from metastasis to distant vital organs and recurrence after treatment. Among the multiple steps of tumor metastasis, the colonization of disseminated tumor cells (DTCs) in distant organs is an inefficient and speed-limited step. Only a small proportion of DTCs can evade powerful immune surveillance, spread from the primary tumor site, survive in new environments, and ultimately colonize distant organs. A series of major advances in tumor metastasis research have revealed complex interactions between DTCs and organ premetastatic niches, crucial for metastatic lesion development. However, the metabolic regulation of DTCs by the organ microenvironment remains unclear.
Limited evidence supports the concept of metabolic regulation in organ niche colonization in preclinical tumor models. In breast cancer lung metastasis models, pyruvate in lung interstitial fluid is absorbed by DTCs, initiating a metabolic cascade that reshapes the extracellular matrix and facilitates lung metastasis niche information. In ovarian cancer models, omental adipocytes provide energy to DTCs through fatty acid oxidation (FAO) or activate survival and proliferation-related signaling pathways, promoting ovarian cancer metastasis to the omentum. These results highlight the organ microenvironment’s novel role in metabolically supporting DTC colonization.
The lung, among the organs prone to solid tumor occurrence, is a common metastatic site. Previous studies in the lung microenvironment have shown that neutral lipids in infiltrating innate immune cells promote tumor metastasis, contributing pre- and post-metastatic niche formation.
This study focused on lung mesenchymal cells (MCs), revealing high levels of intracellular triglyceride (TG) content in both disease-free and tumor-bearing states. The researchers studied TG in one type of lung MCs, adipose fibroblasts, finding that TG protects alveoli from oxidative damage and provides a substrate for lung surfactant synthesis during alveolar development. However, the role of lung MC-derived neutral lipids in pathological processes remains unknown. Therefore, the researchers used a breast cancer mouse model to study the mechanism by which lung MCs regulate breast cancer lung metastasis through neutral lipid metabolism.
In this breast cancer model, researchers found that lung MCs accumulated large amounts of neutral lipids during the pre-metastatic stage, mediated by interleukin-1β (IL-1β)-mediated hypoxia-induced lipid droplet-associated protein (HILPDA). This subsequently inhibited adipose triglyceride lipase (ATGL) activity in lung MCs. Knockout of MC-specific ATGL or HILPDA genes in mice enhanced and reduced breast cancer lung metastasis, respectively, suggesting a metastasis-promoting role of lipid-containing MCs. Mechanistically, lipid-containing MCs transport lipids to tumor cells and natural killer cells (NK) through exosome-like vesicles, leading to tumor cell survival and proliferation and enhancing NK cell dysfunction. Blocking IL-1β alone significantly tumor suppressed tumors and enhanced the immunotherapeutic efficacy of NK cells, mitigating lung metastasis. Overall, lung MCs regulate tumor cell progression and anti-tumor immunity through lipid metabolism, promoting breast cancer lung metastasis.
Although researchers found that neutral lipids, after being transported out of lipid-rich MCs, undergo different fates in tumor cells and NK cells, the underlying molecular mechanisms remain unknown. Additionally, in situ identification of lipid sources, stimulating factors for lipid release from MCs, and lipid transport between cells in different lung environments require further exploration. Despite exosome-like vesicles mediating functional reprogramming of tumor cells and NK cells, the exact metabolites derived from extracellular vesicles remain unidentified. Lipid-containing MCs have only been studied in lung metastasis models, and researchers will continue to explore their role in other contexts such as homeostasis, inflammation, lung cancer, and other lung diseases.
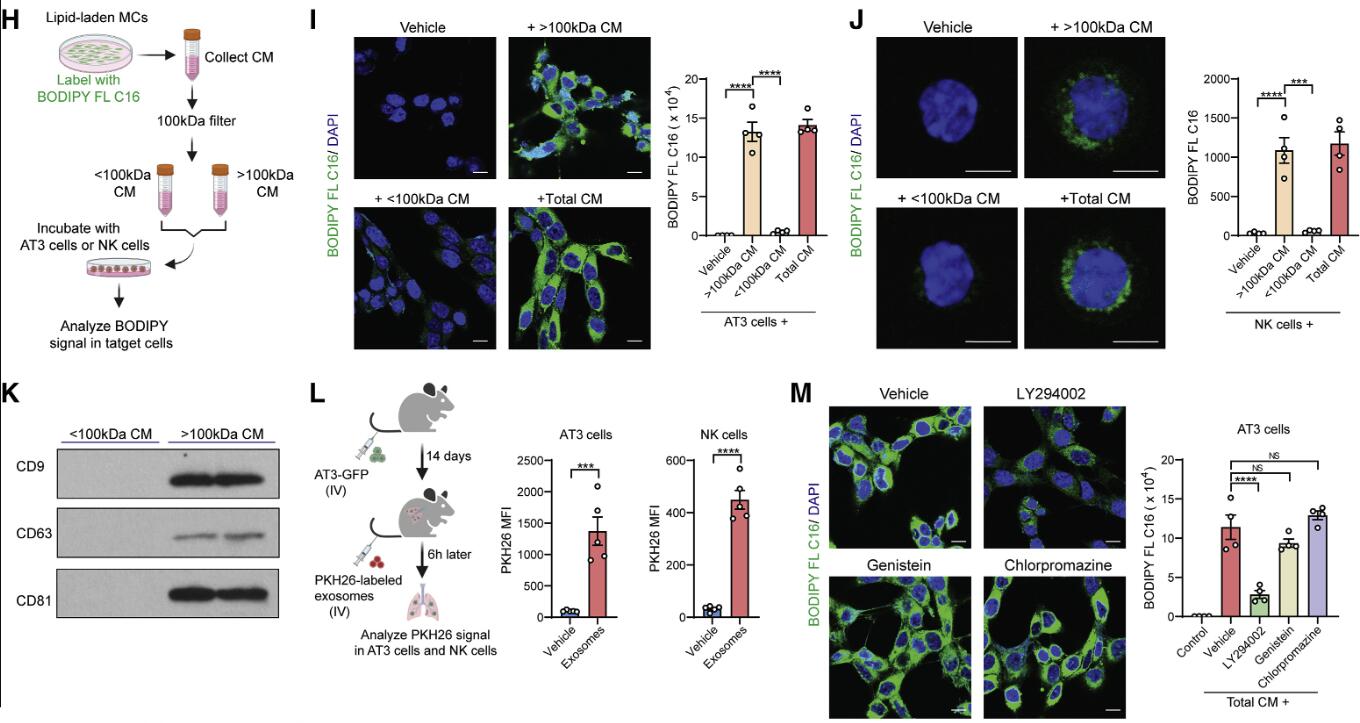 Figure: Lipid-containing MCs transport lipids to tumor cells and NK cells via exosome-like vesicles. Inhibiting lipid transport by lipid-laden MCs through lysosomal pathway inhibitors abolishes metabolic reprogramming of tumor cells and NK cells, thereby reducing metastatic tumor colonization.
Figure: Lipid-containing MCs transport lipids to tumor cells and NK cells via exosome-like vesicles. Inhibiting lipid transport by lipid-laden MCs through lysosomal pathway inhibitors abolishes metabolic reprogramming of tumor cells and NK cells, thereby reducing metastatic tumor colonization.
Reference:
Gong Z, Li Q, Shi J, Liu ET, Shultz LD, Ren G. Lipid-laden lung mesenchymal cells foster breast cancer metastasis via metabolic reprogramming of tumor cells and natural killer cells. Cell Metab. 2022;34(12):1960-1976.e9. doi:10.1016/j.cmet.2022.11.003
Related Services:
Exosome Lipidomics & Metabolomics Services
Breast Cancer-Targeted Exosome Modification Service
Lung Cancer-Targeted Exosome Modification Service
