Small extracellular vesicles (sEVs) play a pivotal role as universal mediators of intercellular communication, emanating from diverse cell types. Despite extensive studies shedding light on the involvement of sEVs in various health and disease contexts, the intricacies of sEV biogenesis and uptake mechanisms have remained elusive due to the limitations of conventional imaging techniques. While traditional fluorescence microscopy has historically been employed for functional studies of sEVs, its restricted resolution has hindered a comprehensive understanding of these processes. Recent advancements in super-resolution microscopy, notably single-molecule localization microscopy (SMLM), offer a promising avenue for unraveling subcellular intricacies at the nanometer scale. Researchers from the University Hospital Essen in Germany delved into the fundamental principles of SMLM, emphasizing the use of suitable fluorophores with exceptional blinking properties. Their work, published in Small on January 12 under the title “Single Molecule Localization Microscopy for Studying Small Extracellular Vesicles,” comprehensively reviews the current application status of SMLM in the realm of sEV biology.
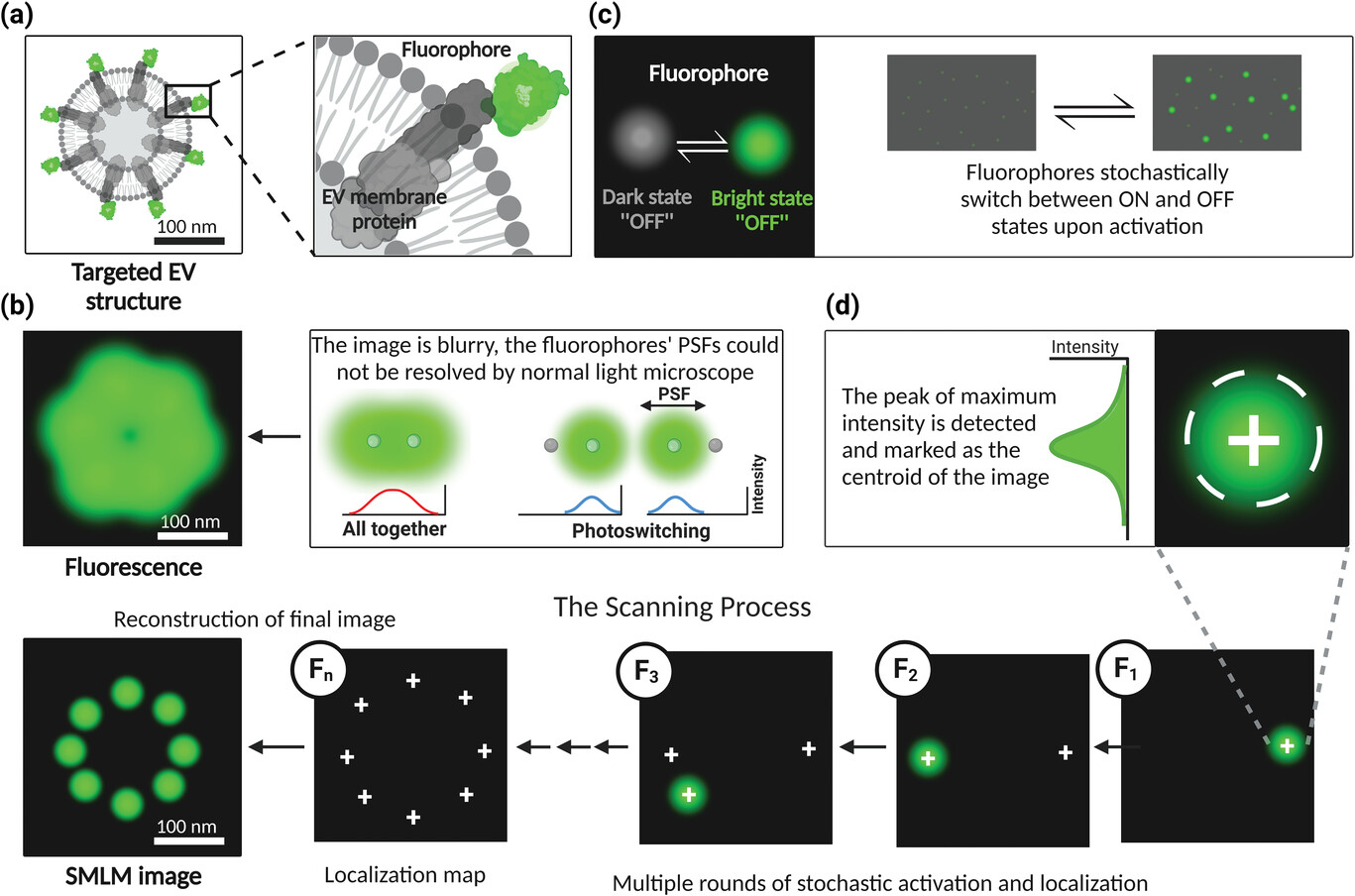 Figure: Principle of single-molecule localization microscopy a) Switchable fluorescent nanogroup-labeled sEVs; b) Fluorescent molecules (green dot) imaged with optical microscopy appear as a blurred point, often called a point spread function (PSF), that extends to multiple pixels in the acquired image; c) SMLM utilizes fluorophores to randomly switch between an active state and one or more inactive states; d) In SMLM, the peak of maximum intensity is detected and marked as the centroid of the image to determine the exact location of the emitting fluorophore.
Figure: Principle of single-molecule localization microscopy a) Switchable fluorescent nanogroup-labeled sEVs; b) Fluorescent molecules (green dot) imaged with optical microscopy appear as a blurred point, often called a point spread function (PSF), that extends to multiple pixels in the acquired image; c) SMLM utilizes fluorophores to randomly switch between an active state and one or more inactive states; d) In SMLM, the peak of maximum intensity is detected and marked as the centroid of the image to determine the exact location of the emitting fluorophore.
Extracellular vesicles (EVs) are lipid bimolecule-bound vesicles secreted into the extracellular space by all types of cells. EVs are roughly divided into three main types, including exosomes (30-150 nm), microvesicles (150-1000 nm) and apoptotic bodies (1-5µm). Acknowledging the heterogeneity of EVs, the MISEV2018 guidelines advocate the use of the term small EVs (sEVs) for vesicles smaller than 200 nm in diameter. Laden with essential cargo biomolecules such as nucleic acids, proteins, and lipids, sEVs emerge as functional mediators of intercellular communication in both health and disease. Notably, sEVs derived from tumor cells harbor disease-specific constituents, reflecting the pathological status and progression. In the tumor microenvironment, sEVs facilitate the transfer of their cargo from tumors to stromal cells, playing pivotal roles in various diseases, including neurodegenerative disorders and infections, while delivering modulators of many biological processes and affecting the immune system.
Over the past decade, research on sEV biogenesis pathways and the role of sEVs in health and disease has grown exponentially. However, studies exploring the interactions of released sEVs and their cargo with cellular biomolecules in distal recipient cells have been severely hampered by the limitations of conventional microscopy techniques. Current studies predominantly utilize traditional confocal microscopy, generating 2D or 3D reconstructed images with Imaris. However, these images, although informative about sEV communication, lack the precision required to discern the exact localization and interaction of sEV-related cargo with cellular biomolecules in the 200-300 nm range. Recent revelations, such as the interaction between EV-DNA from acute myeloid leukemia (AML) and bone marrow-derived mesenchymal stromal cells (BM-MSCs), highlight the need for enhanced imaging techniques. Despite utilizing 2D confocal imaging, the identification of the specific cellular biomolecules in bone marrow mesenchymal stem cells interacting with AML EV-DNA remains elusive. Therefore, the imperative to employ super-resolution microscopy (SRM) becomes evident, particularly single-molecule localization microscopy (SMLM), with innovative labeling strategies enabling optical resolution at the nanometer range.
In the realm of SRM techniques, SMLM stands out for its exceptional resolution and high signal-to-noise ratio, making it particularly advantageous for spatially quantifying the arrangement of single molecules in EV research. However, the current application of SMLM in EV research primarily involves labeling common sEV markers using antibodies linked to different fluorophores. Consequently, the functional exploration of sEVs in distal recipient cells remains very limited. Researchers have endeavored to deploy SMLM to investigate the interaction of sEV-associated DNA cargo with cellular components in distal recipient cells, yet face challenges attributed to the limitations of commonly used fluorophores.
This comprehensive review builds upon prior studies, addressing the pitfalls and limitations of existing labeled fluorophores. It advocates for the adoption of alternative fluorophores with specific scintillation properties in SMLM sEV imaging. The discussion encompasses the translation of SMLM technology for cell imaging into the domain of sEV imaging through diverse labeling strategies, facilitating the study of sEV biogenesis and their biomolecular interactions with distant recipient cells. The review also outlines future advances in live and fixed cell imaging of sEVs at the nanoscale resolution, addressing key questions in sEV biology, including the packaging of diverse biomolecule cargos into sEVs, the uptake of single sEVs, and the molecular interactions of sEVs with specific cellular compartments.
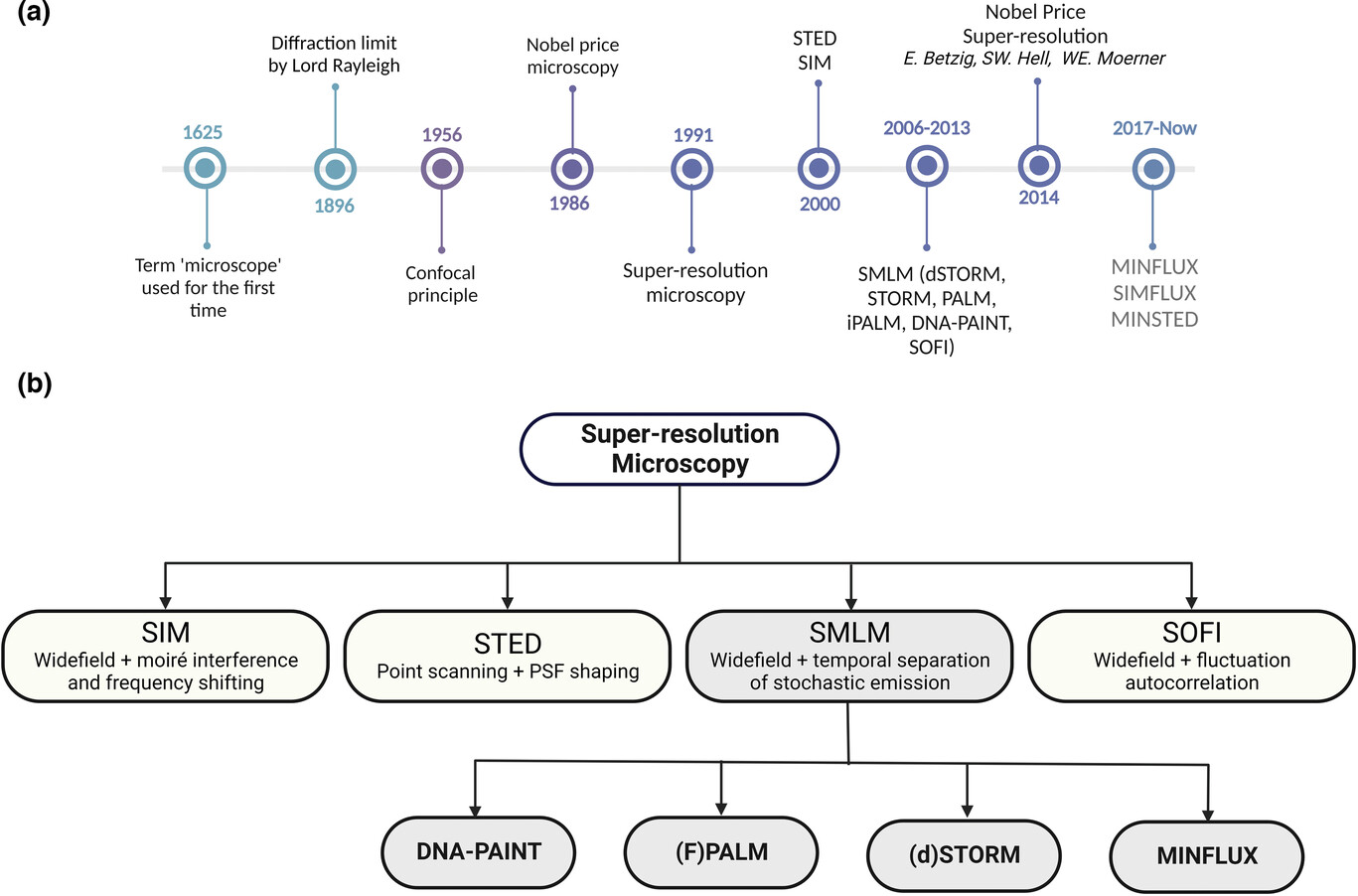 Schematic diagram of super-resolution microscopy technology a) Timeline of major breakthroughs in optical microscopy; b) The SMLM method is based on continuously imaging the sparse signals of fluorophores and calculating their positions from the obtained diffraction patterns.
Schematic diagram of super-resolution microscopy technology a) Timeline of major breakthroughs in optical microscopy; b) The SMLM method is based on continuously imaging the sparse signals of fluorophores and calculating their positions from the obtained diffraction patterns.
Reference:
Ghanam J, Chetty VK, Zhu X, et al. Single Molecule Localization Microscopy for Studying Small Extracellular Vesicles. Small. 2023;19(12):e2205030. doi:10.1002/smll.202205030
Related Services:
