Effective and targeted drug delivery remains a significant challenge in modern medicine. Researchers are employing biochemical engineering approaches to repurpose extracellular vesicles (EVs) as drug delivery vehicles. Studies have demonstrated that displaying targeting ligands such as GalNAc on the surface of EVs and utilizing HaloTag fused to protein anchors enriched on engineered EVs can successfully target human primary hepatocytes. Moreover, studies decorating EVs with antibodies that recognizing the GLP1 cell surface receptor have shown improved targeting of EVs to cells overexpressing this receptor. The study has also enhanced the efficiency of Cre recombinase loading into the EVs chamber. These findings suggest that EVs can be engineered to improve payload loading and enable specific cell targeting, transforming them into customized drug delivery vehicles. The relevant content was published online onOctober 22 in the international advanced materials academic journal Advanced Science titled “Creating Designer Engineered Extracellular Vesicles for Diverse Ligand Display, Target Recognition, and Controlled Protein Loading and Delivery.”
Researchers describe the challenges of targeted delivery of drug types such as enzymes, antibodies, peptides, and nucleic acids to specific treatment sites and propose potential solutions utilizing extracellular vesicles (EVs) as drug encapsulation platforms. EVs are natural nanosized lipid bilayer particles capable of delivering bioactive molecules, inducing functional responses, and participating in intercellular communication. They are released by nearly all cell types and internalized by nearby or distant receptor cells. Compared to synthetic nanoparticles, EVs offer unique advantages, including excellent biocompatibility, stability, and low immunogenicity. They protect their cargo during circulation and their surface provides naturally occurring modification sites that enhance their functionality. However, despite these remarkable features of EVs, challenges remain, such as rapid clearance from circulation, intrinsic targeting requiring functionalization, and limited loading capacity.
Despite the breakthroughs in EV engineering, delivering EVs specifically to target cell populations remains a challenge. To overcome this, researchers modify EV surfaces to display various targeting molecules recognized by specific cells. For example, antibodies or antibody fragments have been integrated into EV surfaces, offering versatility as antibodies can be designed for any target. However, the use of monoclonal antibodies as targeting molecules is limited due to their large size and complexity.
Most cell engineering strategies focus on directing targeting peptides and proteins to EV protein sorting domains, such as Lamp2b, tetraspanin, and PTGFRN. However, incorporating more complex molecules using this approach is challenging. An alternative method involves modifying the EV surfaces through cross-linking reactions, such as azide-alkyne cycloaddition or click chemistry, to attach targeting molecules covalently. This technique is suitable for introducing large molecules, small molecules, sugars, or polysaccharides onto EV surfaces. Some targeting peptides, such as epidermal growth factor or those with high affinity for integrin αvβ3, have been successfully introduced using this method.
Another obstacle in utilizing EVs as drug delivery vehicles is delivering drug payloads and proteins into recipient cells’ cytoplasm. Studies have demonstrated that light- and small-molecule-induced dimerization systems can increase cargo protein load, such as SpCas9 and Cre recombinase, within EVs. However, releasing proteins inside EVs during genetic engineering remains challenging and requires additional stimulation.
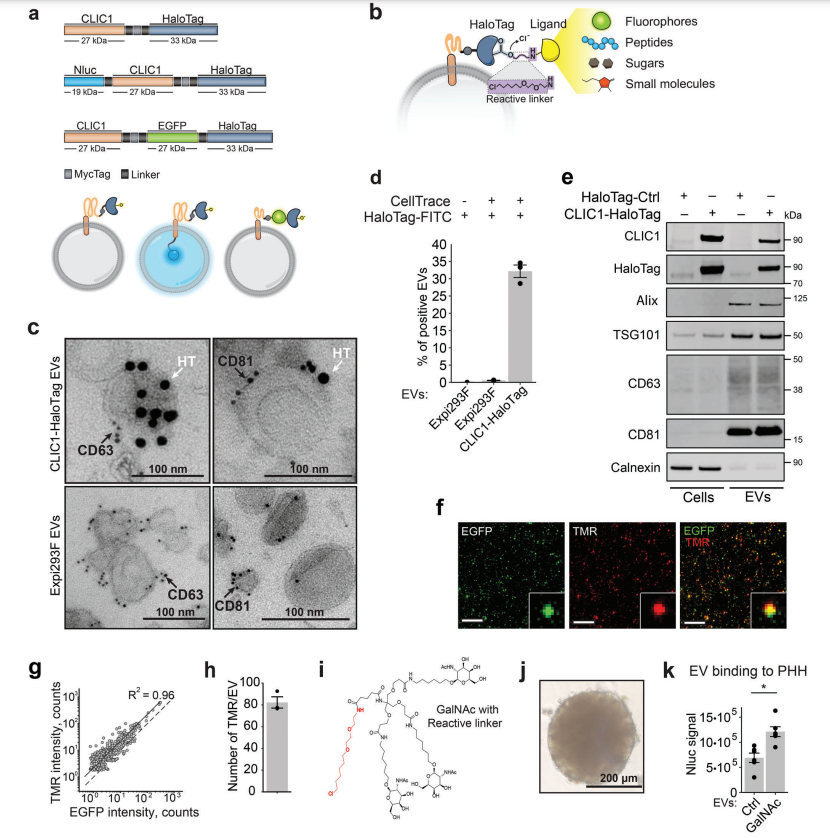
In this study, researchers employed a variety of state-of-the-art genetic engineering methods to modify EV compositions, enhancing cell targeting and loading. Specifically, EV surfaces were decorated by attaching a vesicle-anchored protein fused to a modified haloalkane dehalogenase protein tag (HaloTag). This tag covalently binds to synthetic ligands carrying chlorinated alkane linkers, allowing the introduction of various molecular effectors such as fluorescent dyes, peptides, sugars, and small molecules on to EV surfaces. The researchers used this system to decorate purified EVs with trivalent N-acetylgalactosamine (GalNAc) and demonstrated that these engineered EVs preferentially bind to primary human hepatocytes. The research also developed a complementary system to display antibodies on EV surfaces.
Additionally, two protein engineering approaches were employed to enhance Cre recombinase loading and protein release into EV lumens during genetic engineering. These modified EVs deliver functional Cre into recipient cell cytoplasm after treatment with endosomal escape enhancers. Importantly, these modifications do not alter EVs’ fundamental properties. When injected into mice, the engineered EVs were well tolerated, exhibiting no detectable liver toxicity. Overall, these findings underscore the significant potential of engineered EVs as unique protein-targeting therapeutic drug delivery systems.
Reference:
Ivanova A, Badertscher L, O’Driscoll G, et al. Creating Designer Engineered Extracellular Vesicles for Diverse Ligand Display, Target Recognition, and Controlled Protein Loading and Delivery [published online ahead of print, 2023 Oct 22]. Adv Sci (Weinh). 2023;e2304389. doi:10.1002/advs.202304389
Related Services:
