A recent review was published in Nature Reviews Rheumatology delves into the promising therapeutic applications of mesenchymal stromal cell extracellular vesicles (EVs) in rheumatic diseases.
Rheumatic diseases comprise a diverse range of conditions affecting joints, bones, muscles, and connective tissues. Conditions like osteoarthritis (OA) and rheumatoid arthritis (RA) rank among the leading causes of severe global disability, imposing a significant economic burden.
In severe cases, surgical interventions become necessary, but complexities like systemic involvement often lead to suboptimal outcomes, especially in OA. Current treatments mainly focus on managing symptoms, often relying on medications like NSAIDs for inflammation and pain control, alongside physical therapy and assistive devices to enhance joint mobility and function. While these approaches offer symptomatic relief, they rarely impede the disease’s progression.
Cell therapy using mesenchymal stem cell (MSC) therapy presents a potential solution. MSCs exhibit potent immunosuppressive and regenerative effects, sparking extensive research into their efficacy against rheumatic diseases. Despite promising outcomes in clinical trials, the use of MSCs is hindered by donor heterogeneity and batch variations, affecting their predictability. To overcome these challenges, researchers are exploring MSC-derived extracellular vesicles (MSC-EVs). Interest in EVs has surged due to their biological content, demonstrating their ability to mediate MSC effects, particularly in OA, both locally and systemically.
This review not only explores the sources of MSC-EV production heterogeneity but also showcases the progress in utilizing MSC-EVs to treat rheumatic diseases, with a specific focus on OA and RA. It also provides insights into their application in other degenerative diseases. Additionally, the review emphasizes the crucial need for optimizing EV production methods to enhance isolation yields biotherapeutic potency. Preclinical data on MSC-EVs’ efficacy in treating rheumatic diseases are discussed in detail, offering a comprehensive overview of their potential.
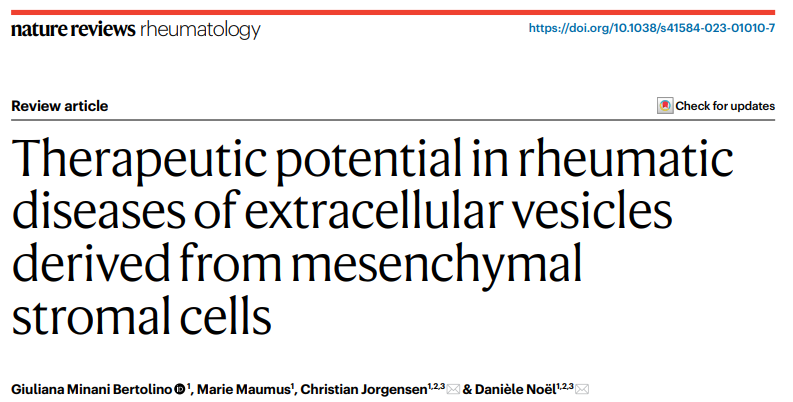
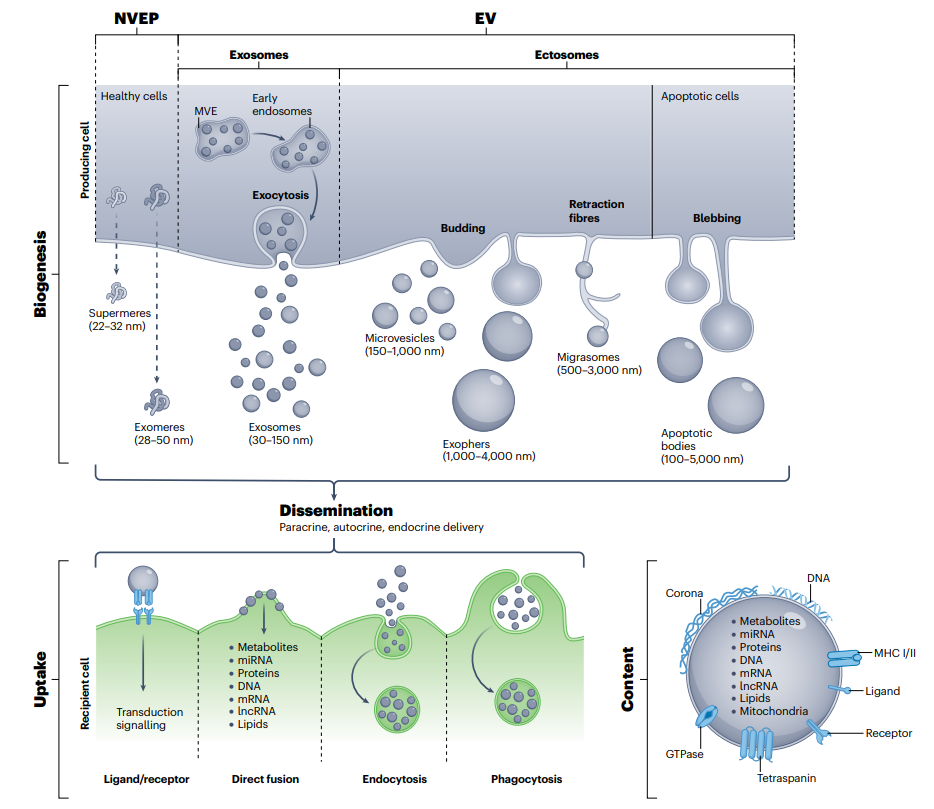 Biogenesis, Uptake and Content of Extracellular Vesicles
Biogenesis, Uptake and Content of Extracellular Vesicles
As our population ages and high-intensity physical activities become more prevalent among young and middle-aged individuals, the incidence of rheumatic diseases like rheumatoid arthritis and osteoarthritis, and articular cartilage damage leading to osteochondral damage is on the rise. Current treatments primarily revolve around managing pain and improving joint function to enhance patients’ quality of life. However, the need for definitive curative strategies has never been more crucial. Over the past two decades, scientists have been exploring the therapeutic value of mesenchymal stem cells (MSCs) due to their remarkable regenerative capabilities, primarily attributed to their secretion of paracrine factors. Notably, many of these beneficial factors are encapsulated within in extracellular vesicles (EVs), which mimic the essential functions of their parent cells. MSC-derived EVs exhibit anti-inflammatory, anti-apoptotic, and regenerative properties, making them highly valuable in the realm of rheumatic disease treatments. Research on EVs has gained immense traction because they offer a cell-free therapeutic approach characterized by lower immunogenicity and easier administration. Remarkably, MSC-derived EVs have the ability to rescue the pathogenic phenotype of chondrocytes and exert protective effects in animal models of rheumatic diseases. In the pursuit of advancing extracellular vesicle (EV) therapy, identifying the optimal cell source is paramount to harnessing their desired biological effects for each disease. This meticulous approach ensures that EV production and isolation processes are meticulously optimized, coupled with pre-isolation and post-isolation modifications. These strategies are crucial in maximizing the disease-repairing potential of EVs.
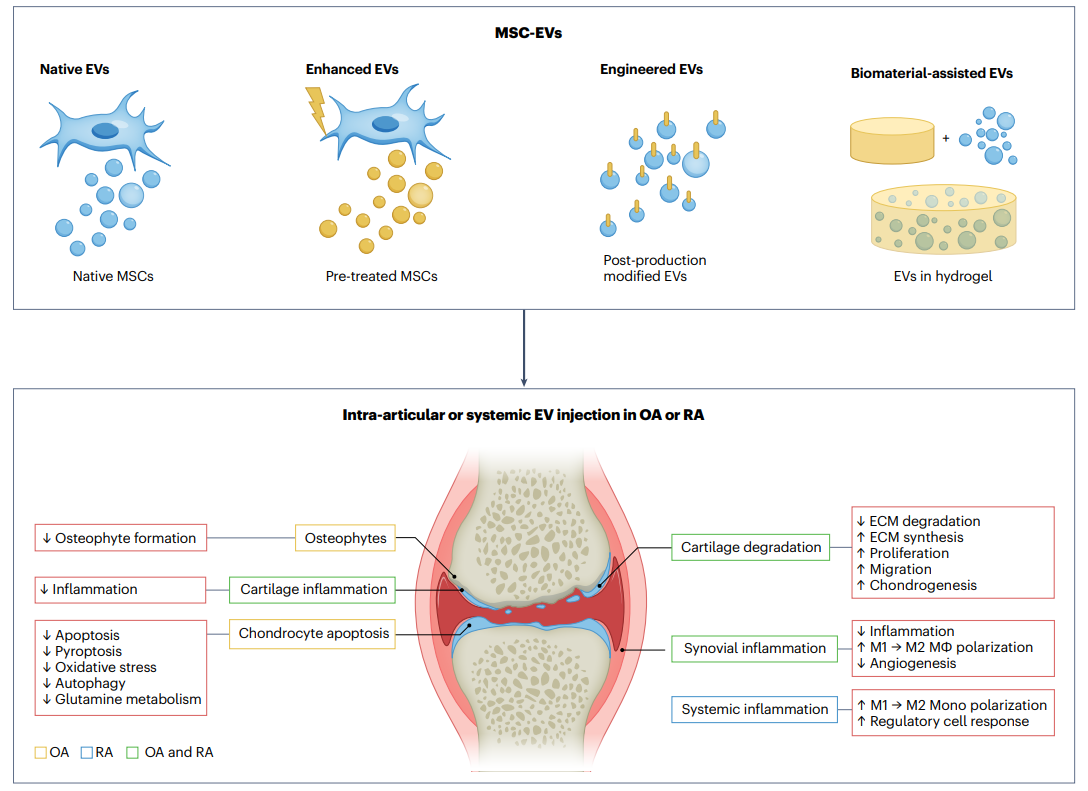 Applications of MSC-derived EVs in OA and RA
Applications of MSC-derived EVs in OA and RA
Over the last decade, the realm of clinical EV applications has witnessed groundbreaking developments, especially in identifying MSC-EVs as innovative therapeutics. Within this emerging field, compelling evidence supports the use of EV-based therapies in rheumatic diseases, notably OA and RA. These EVs play a pivotal role in regulating and preventing these conditions by addressing both local and systemic inflammation, while restoring a healthy chondrocyte phenotype. Yet, treating RA presents unique challenges due to its systemic nature and the occurrence of multiple joint injuries. To navigate these complexities, researchers must leverage insights from recent MSC-based clinical trials. Strategies such as patient stratification based on treatment response and the identification of predictive markers are essential avenues for refining future treatments. In the ongoing quest for effective treatments, a phase I clinical trial is underway, exploring MSC-EV-based therapy for OA. To enhance the relevance of these trials, investigating optimal dosage and treatment timing in large animal models, closer to the human situation than mouse models, is imperative. An intriguing prospect lies in the use of allogeneic MSC-EVs or packaged MSC-EVs, allowing for repeated doses and offering a more enduring solution than maternal MSCs. These EVs exhibit exciting potential, demonstrating their ability to reduce inflammation and enhance cell survival and synthetic activity in various joint regions. This presents a novel therapeutic approach in the realm of rheumatic diseases. However, continuous research is essential to unravel the full potential of MSC-EVs. Gaining a comprehensive understanding of their functions and discovering ways to enhance their effectiveness in regenerative medicine is pivotal. These efforts are crucial in slowing disease progression and transforming the landscape of rheumatic disease treatments.
Reference:
Bertolino GM, Maumus M, Jorgensen C, Noël D. Therapeutic potential in rheumatic diseases of extracellular vesicles derived from mesenchymal stromal cells. Nat Rev Rheumatol. 2023 Sep 4. doi: 10.1038/s41584-023-01010-7. Epub ahead of print. PMID: 37666995.
Related Services:
Synovial Fluid-Derived Mesenchymal Stem Cells (SF-MSCs)-Targeted Exosome Modification Service
