Non-alcoholic fatty liver disease (NAFLD) is strongly linked to various cancers, including liver, breast, prostate, pancreatic, and colorectal cancer, surpassing its association with obesity alone. Patients with fatty liver disease exhibit a higher incidence and recurrence of colorectal cancer (CRC) liver metastases compared to those withoutfatty liver disease. Liver metastasis stands as the primary cause of death in CRC patients. While fatty liver promotes liver metastasis, the underlying mechanisms remain elusive. A recent article in Cell Metabolism shed light on this topic, highlighting the role of extracellular vesicle (EV) EV-microRNAs, YAP signaling, and an immunosuppressive microenvironment induced by fatty liver in promoting the growth of colorectal cancer liver metastases.

Colorectal cancer ranks as the third most prevalent malignant tumor globally and the second leading cause of cancer-related death, claiming approximately 900,000 lives annually. The liver serves as the primary site for CRC metastasis due to the unique anatomical connection between the intestine and liver through the portal vein. Liver-specific metabolic and immune microenvironments further facilitate CRC liver metastasis, eventually, affecting 70% of CRC patients and standing as the leading cause of mortality.
Obesity and non-alcoholic fatty liver disease (NAFLD) emerge as pivotal risk factors for CRC. With more than 650 million adults worldwide grappling with obesity, the surge in NAFLD has become intricately linked to the obesity epidemic. Mounting epidemiological evidence underscores that fatty liver significantly heightens the incidence of liver metastasis and local recurrence of CRC, thereby worsening the overall prognosis. Notably, fatty liver disease enhances metastatic liver tumor growth in animal models of CRC liver metastases by reshaping the liver’s inflammatory and immune microenvironment. These findings intimate that the mechanisms of metastasis might vary due to the heterogeneous in the tumor microenvironment (TME) in fatty liver, potentially explaining the diverse responses to cancer treatments among patients. Consequently, the management of metastases in CRC patients might necessitate tailored approaches based on the presence or absence of fatty liver disease. Understanding the molecular intricacies of metastasis in patients with fatty liver disease becomes imperative to effectively strategize their management.
Extracellular vesicles (EVs) play a significant role in intercellular communication, containing vital macromolecules such as microRNAs (miRNAs), lipids, and proteins. The regulation of EV production and secretion in the extracellular space is governed by sphingomyelinylcholine synthesis and Rab proteins, including Rab27a and Rab27b. In melanoma, Rab27a mediates EV production and transfer, essential for lipid overload-enhanced EV secretion. Notably, in non-alcoholic fatty liver disease (NAFLD), hepatocyte-derived EV production increases, leading to alterations in miRNA content. Previous studies have demonstrated the role of primary tumor-derived EVs in promoting premetastatic niche formation and pro-metastatic inflammatory responses in the liver. However, the specific contribution of liver-produced EVs under NAFLD conditions to the hepatic environment predisposing and promoting CRC liver metastasis remains unexplored.
The Hippo pathway effector, YAP, regulates oncogenic transcription factors, like transcription enhancer binding domains (TEADs). Phosphorylation of YAP byMST1/2 and LATS1/2 causes its cytoplasmic localization and degradation, inhibiting its activity. Unphosphorylated YAP trans-locates to the nucleus, activating TEAD transcription factors and promoting cancer growth, invasion, and metastasis. G protein-coupled receptors, mechanical stimulation, cell adhesion, and integrin signaling regulate YAP activity in a phosphorylation-dependent manner, but YAP activity is also subject to post-transcriptional regulation by miRNAs. Oncogenic YAP activity significantly influences cancer cell behavior and the surrounding immune cells. The loss of LATS1/2 kinase enhances anti-cancer immunity, while high YAP activity recruits M2 tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells, or upregulates programmed cell death 1 ligand (PD-L1) expression, inducing an immunosuppressive microenvironment.
This study reveals that EVs from fatty liver harbor pro-oncogenic miRNAs, shaping a pre-metastatic and pro-metastatic liver microenvironment conducive to CRC liver metastasis. Our findings demonstrate that EV-mediated transfer of pro-oncogenic miRNAs from fatty liver hepatocytes to metastatic cancer cells inhibits LATS2, resulting in increased YAP activity. Elevated YAP activity fosters CRC liver metastasis by enhancing recruitment of M2-tumor-associated macrophages (TAMs) and depleting CD8 T cells, establishing an immunosuppressive milieu. Consequently, NAFLD gives rise to a complex metastatic TME that promotes CRC liver metastasis. Moreover, the escalating prevalence of NAFLD might account for the differential responses to cancer treatment observed in patients with CRC and liver metastases.
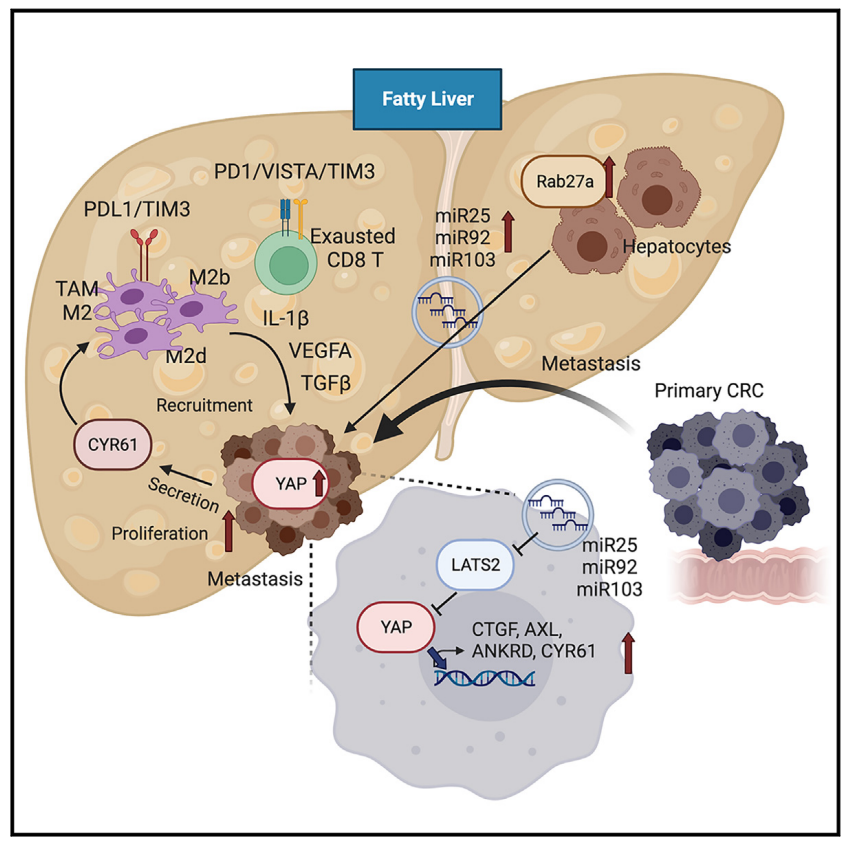
This study demonstrates that extracellular vesicles (EVs) derived from hepatocytes in fatty liver enhance the progression of CRC liver metastasis by promoting oncogenic Yes-associated protein (YAP) signaling and creating an immunosuppressive microenvironment. Fatty liver upregulates Rab27a expression, thereby promoting EV production from hepatocytes. In the liver, these EVs transfer microRNAs that regulate YAP signaling into cancer cells, increasing YAP activity by inhibiting LATS2. In CRC liver metastases with fatty liver, increased YAP activity promotes cancer cell growth and an immunosuppressive microenvironment, leading to M2 macrophage infiltration through CYR61 production. Patients with CRC liver metastases and fatty liver exhibit elevated nuclear YAP expression, CYR61 expression, and M2 macrophage infiltration. The data indicate that fatty liver-induced EV-microRNAs, YAP signaling, and the immunosuppressive microenvironment promote the growth of CRC liver metastases.
Reference:
Wang Z, Kim SY, Tu W, et al. Extracellular vesicles in fatty liver promote a metastatic tumor microenvironment. Cell Metab. 2023;35(7):1209-1226.e13. doi:10.1016/j.cmet.2023.04.013
Related Services:
