Recent studies have revealed that extracellular vesicles (EVs) surface molecules are often accompanied by glycan or glycosylation modifications. Researchers from the Institute of Chemistry at the Slovak National Academy of Sciences have published a research-based review that discusses the role of glycosylation in EV formation, loading, and release. They describe methods for glycan identification and glycan-based analysis to capture EVs and achieve high-sensitivity detection. In addition, the researchers provide detailed insights into EV glycans and glycan-processing enzymes as potential biomarkers, therapeutic targets, or tools for regenerative medicine applications. This relevant content was published online on June 10th in Biotechnology Advances, an international academic journal in the field of bioengineering, titled “Glycosylation in Extracellular Vesicles: Isolation, Characterization, Composition, Analysis and Clinical Applications.”
An outline of this review includes:
- Glycans’ crucial role in EV formation, loading and release.
- Formation of a biomolecular corona with a thickness of 5-70 nm by the glycan component of EVs.
- Detection of 1 extracellular vesicle per microliter under anhydrous conditions using the mannan method.
- Primarily analyzing glycans in extracellular vesicles are primarily analyzed using lectin microarrays that incorporate up to 45 lectins.
- Promising applications of glycans in extracellular vesicles have promising applications as biomarkers.
Extracellular vesicles (EVs) are lipid bilayer-bound vesicles secreted into the extracellular space by different cells. EVs play pivotal roles in intercellular communication, gene expression regulation, reproduction, cell development and proliferation, wound healing, waste management, metabolic regulation and reprogramming, signal transduction, immune response, apoptosis, and cancer initiation and progression. Two primary subpopulations of EVs exist: including exocytic particles (ectosomes, 50-10,000 nm) formed by membrane budding on the plasma membrane, and exosomes (30-150 nm) budding inwardly through endosomal membranes in cells. Moreover, other types of EVs have also been described, including migratory bodies (500–3,000 nm), secretory autophagosomes and autophagic endosomes, exosomes (1,000–10,000 nm) and apoptotic bodies (50-5,000 nm), among others.
Traditional EV isolation methods encompass ultracentrifugation, particle size separation chromatography, precipitation, extraction, ultrafiltration, immunoaffinity capture, microfluidics, and charge-based separation techniques. An increasingly utilized method is bio-affinity capture of EVs using antibodies against EV surface receptors, such as CD9 immobilized on magnetic beads (MB). Direct comparisons have shown that covalently immobilized antibodies on MBs outperform streptavidin-conjugated MBs coated with biotin-modified antibodies. The researchers suggest adopting a “cocktail” isolation strategy that combines multiple isolation methods for high purity and isolation yield. This review also delves into MB-based EV isolation strategies and affinity-based methods, and other nanoparticles (NPs) used for EV isolation, including glycan-based methods.
Maintaining the structural integrity of isolated EVs is crucial. Traditional buffers such as phosphate-buffered saline (PBS) are discouraged for EV storage, even for diluted EVs, as EV dispersion in PBS is unstable. Instead, the authors recommend dispersing EVs in PBS supplemented with trehalose and human serum albumin, followed by EV processing and long-term storage at -80°C. As EVs are a diverse particle type, a range of instrumental techniques is required for their study, including photonics and biophotonics (Raman spectroscopy, Fourier transform infrared spectroscopy, surface plasmon resonance, flow cytometry, fluorescence imaging), and other techniques (electron microscopy, atomic force microscopy, nanoparticle tracking analysis, nuclear magnetic resonance, dynamic light scattering, mass spectrometry, microfluidics, etc.). These techniques not only facilitate the study of EV composition, size, and number but also enable the identification of specific markers. While fluorescent dyes can visualize EVs, labeling EVs requires optimization, as some commonly used fluorochromes only label a small fraction of EVs, and these dyes tend to aggregate, not associating effectively with EVs.
Moreover, membrane-bound surface proteins, known as glycoproteins, can be effectively employed for EV isolation and detection. For example, prostate-specific membrane antigen, a well-known prostate cancer biomarker, is notably enriched in prostate-derived exosomes found in urine. Similarly, other cancer biomarkers like human epidermal growth factor receptor 2 (HER2 or CD340 or erbB-2) can serve therapeutic purposes, although not all cells express this receptor. Beyond proteins and lipids, the surface of EVs showcases a diverse array of glycoconjugates, encompassing O-glycans, N-glycans, glycolipid gangliosides, and other glycan types, all suitable for EV isolation. Leveraging the principle of glycans on EV surface, lectin-based affinity chromatography has been employed successfully to isolate of intact EVs (124-134 nm) produced by cell lines from sponge-like polymers. Notably, studies have demonstrated that even Evs originating from the same cell type and size exhibit heterogeneity in the glycans expressed on their surfaces, enabling the classification of EVs based on their surface glycan features.
Within this review, the researchers comprehensively outline the pivotal role of glycans in the formation, loading, and release of EVs, typically ranging in size from 100-200 nm. Glycans, as components of EV membranes, generate biomolecular coronas with a thickness ranging from 5 to 70 nm, offering an effective avenue for EV separation, particularly through magnetic particles. The review presents compelling evidence supporting the utilization of EV glycans in liquid biopsies, therapeutic applications, and regenerative medicine. The emergence of glycans in diverse fields is anticipated, particularly as our understanding of the biomolecular corona present on EVs expands. Research into the clinical applications of EV glycans is experiencing exponential growth. The robustness of EV glycan-based diagnosis necessitates validation in hundreds of samples, ensuring reliability when the AUC value exceeds 0.8. While various nanoparticles, especially magnetic nanoparticle types, have been employed for EV isolation and characterization, it is foreseeable that nanoparticles play a pivotal role in highly sensitive and robust glycan analysis, facilitating the direct visualization of on-membrane and EV-bound glycans within a molecular corona.
Looking ahead, EV glycans are poised to demonstrate their potential as clinical biomarkers for numerous diseases, including various forms of cancer. Thus far, the direct in situ visualization of glycans containing sialic acid residues on EVs has only been achievable through metabolic glycan labeling. A significant future advancement will encompass tools enabling the in situ visualization of other carbohydrates types(such as caramel) commonly associated with various diseases. A current major development involves the ability to determine whether a protein of interest, such as PD-L1, contains glycans containing sialic acid residues. Two approaches have surfaced for tailoring the glycosylation of EVs to meet specific requirements. The first approach involves the modification of EVs using hyaluronic acid, combined with metabolic glycan labeling, enabling the modified EVs to target cells within selected tissues. The second approach entails the introduction of glycosylation domains into receptors present on EVs, followed by glycosylation of these domains using glycan processing enzymes (glycosyltransferases or glycosidases).
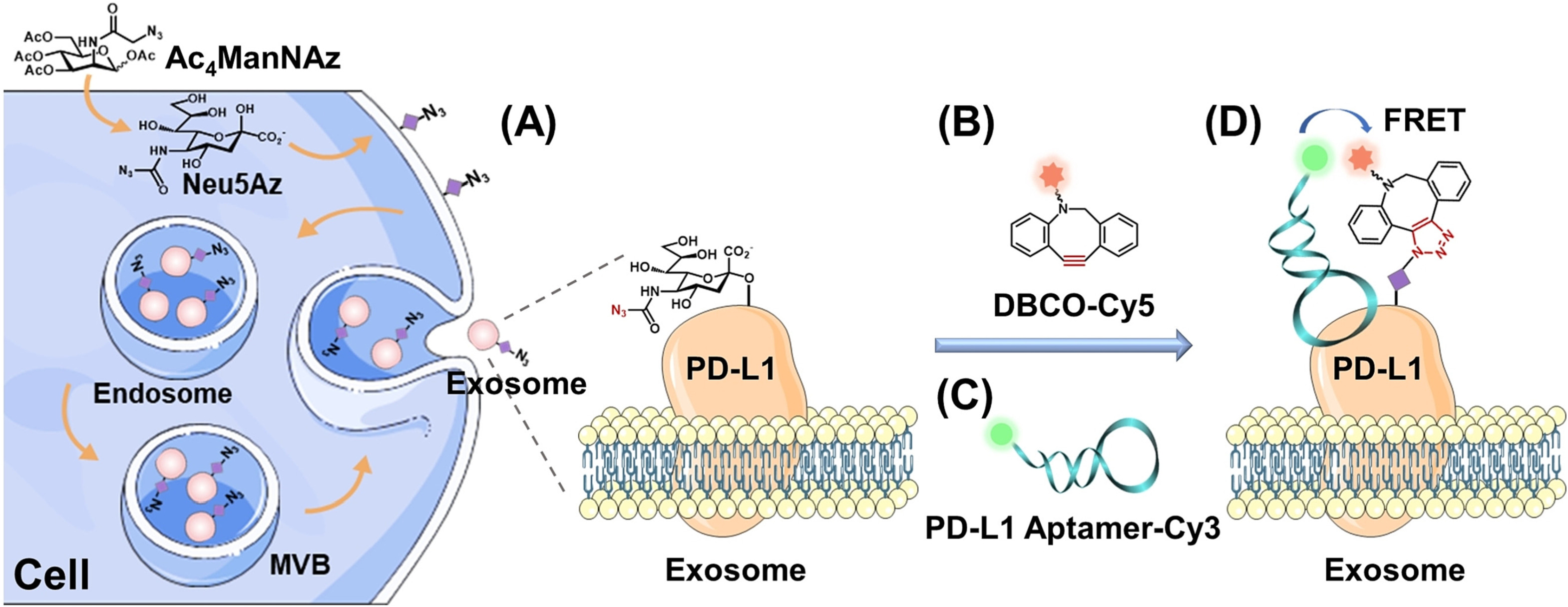 Multiple approaches used to identify glycan modifications of PD-L1 on the EV surface
Multiple approaches used to identify glycan modifications of PD-L1 on the EV surface
References:
Vrablova V, Kosutova N, Blsakova A, Bertokova A, Kasak P, Bertok T, Tkac J. Glycosylation in extracellular vesicles: Isolation, characterization, composition, analysis and clinical applications. Biotechnol Adv. 2023 Oct; 67:108196. doi: 10.1016/j.biotechadv.2023.108196. Epub 2023 Jun 10. PMID: 37307942.
Related Services:
