Bardet-Biedl syndrome (BBS) is a classic ciliopathy. In this disease, mutations in genes essential for maintaining cilia function lead to cellular dysfunction in a variety of cell types, resulting in obesity, polydactyly, renal failure, and blindness. Many of these mutations disable the function of a protein complex called BBSome, which acts as a cargo adapter for the intraflagellar transport (IFT) complex, expanding the range of IFT cargo in ciliary transport and regulating the movement of cargo proteins into and out of the cilia.
Blindness in BBS is caused by dysfunction and loss of retinal photoreceptor cells. Mutations in BBS10, which accounts for approximately 21% of all BBS cases, encode a chaperone protein that is essential for the assembly of the BBSome, a cargo adapter critical for ciliary transport. Loss of BBSome function in the eye results in reduced photoreceptor sensitivity to light, photoreceptor cilia aberrations, cilia transport dysfunction, and photoreceptor cell death. Retinal cone photoreceptors lacking BBS10 have innately lower electrical function in retinal electroretinography.
Recently, researchers from the University of Lowa published an article in Molecular Therapy: Nucleic Acids titled “Subretinal gene therapy delays vision loss in a Bardet-Biedl Syndrome type 10 mouse model,” which found that subretinal gene therapy has significant benefits in delaying vision loss.
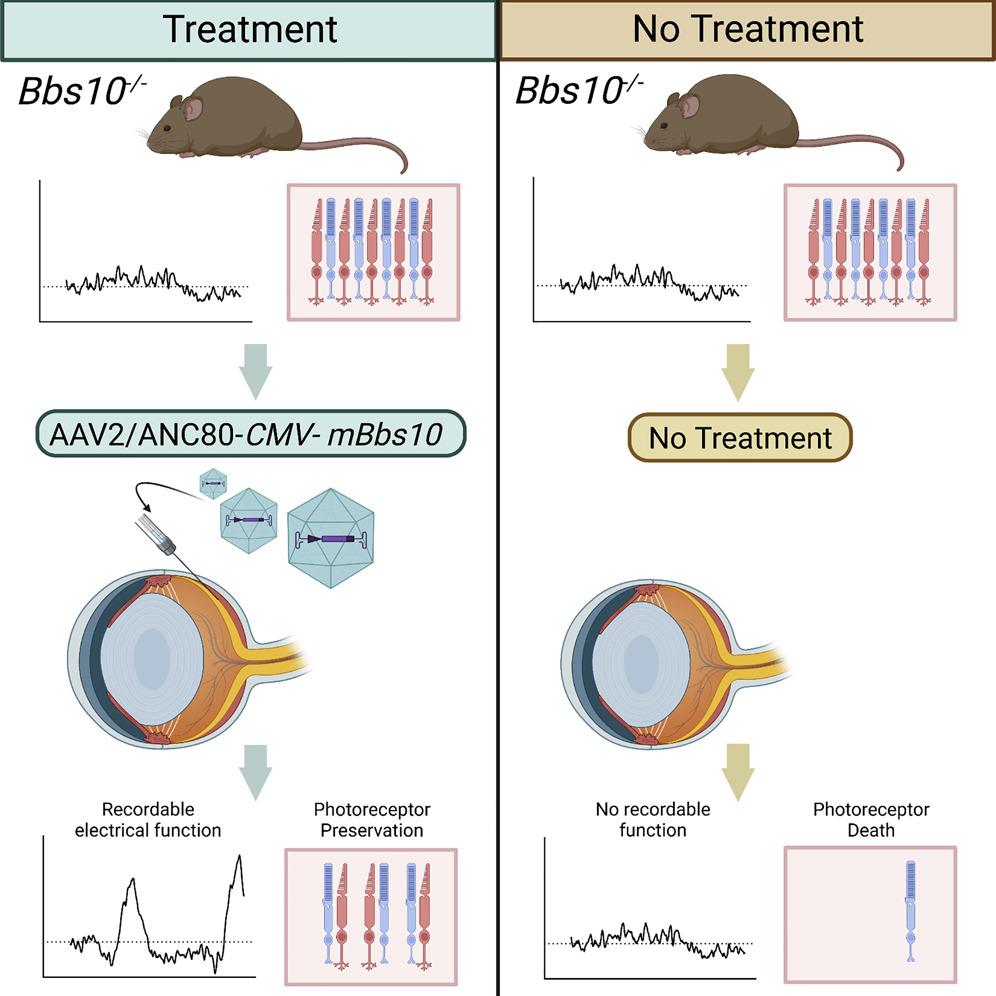
In this study, the investigators administered gene enhancement therapy by subretinal injection of a viral vector to deliver the coding sequence of the mouse BBS10 gene to treat retinal degeneration in a BBS10 mouse model. Long-term efficacy was assessed by measuring the electrical function of the retina over time, imaging the treated area to visualize cell survival, performing a visually guided swim test to measure functional visual acuity, and performing retinal histology.
Subretinal gene therapy delayed the death of photoreceptor cells and preserved retinal function in the treated eyes. Notably, following gene enhancement, the retinal cone photoreceptor cells recovered their electrical function.
Although many unanswered questions remain, the investigators demonstrates that subretinal gene therapy in the BBS10 mouse model delays the loss of vision in BBS. Addressing challenges in long-term efficacy attenuation, determining the feasibility of late-stage treatment, deepening the understanding of dose-response and interspecies dose switching, and taking initial clinical conversion steps are the top priorities at this time.
Reference
1. Hsu, Ying, et al. “Subretinal gene therapy delays vision loss in a Bardet-Biedl Syndrome type 10 mouse model.” Molecular Therapy-Nucleic Acids 31 (2023): 164-181.
