Cell growth is strictly regulated by tumor suppressor genes (TSGs), so that cells divide only when absolutely necessary and then respond to appropriate external signals, such as growth factors. In addition to controlling proliferation, TSGs are also involved in preventing cell migration and invasion of other tissues, as well as stimulating apoptosis when cells experience cellular stress (e.g., DNA damage). If the latter is not controlled, it may lead to the introduction of mutations and dysregulation of the cell cycle.
When TSG function is lost, critical cellular processes become dysregulated, and cells may proliferate uncontrollably, fail to initiate an apoptotic response to damage, or begin to invade and metastasize to different parts of the body through the basement membrane. Therefore, TSGs are an important set of genes in the context of cancer pathogenesis and treatment.
Recently, researchers from the University of Liverpool published a paper in Molecular Therapy: Nucleic Acids entitled “Modulating the expression of tumor suppressor genes using activating oligonucleotide technologies as a therapeutic approach in cancer”. The study summarizes the use of activating oligonucleotide technologies to modulate the expression of oncogenes as a therapeutic approach in cancer.
Tumor suppressor genes (TSGs) are frequently downregulated in cancer, resulting in dysregulation of the pathways they control. A continuum model of tumor suppression suggests that even subtle changes in TSG expression, for example, driven by epigenetic modifications or copy number alterations, may result in loss of gene function and phenotypic effects.
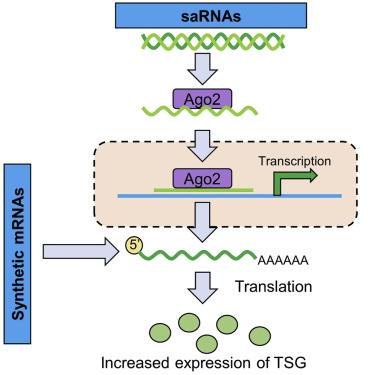
This approach to exploring tumor suppression provides opportunities for alternative therapies that may be able to restore TSG expression to normal levels, such as oligonucleotide therapies. Oligonucleotide therapies involve the administration of exogenous nucleic acids to regulate the expression of specific endogenous genes. The authors review two activated oligonucleotide therapies, small activating RNAs (saRNAs) and synthetic mRNAs, which are novel approaches to increase the expression of TSGs in tumors.
TSGs are typically downregulated in cancer, and although this idea has gained much attention in the scientific community, efforts to upregulate or reactivate TSGs have so far remained relatively futile. However, emerging classes of therapies, such as oligonucleotide therapies, offer advantages over traditional small molecule drugs because they are able to act at the genetic level, rather than by targeting proteins.
Oligonucleotide therapies have the potential to combine with small molecules or other oligonucleotides, leading to synergistic effects. saRNAs and nucleoside-modified RNAs are two classes of oligonucleotide therapies that have the potential to upregulate the expression of target genes or, in the case of synthetic mRNAs, replace lost or misdirected proteins in cancer. saRNAs offer better benefits than synthetic mRNAs in terms of size and delivery ability; however, they are inappropriate therapeutic options for patients with mutated target genes, as upregulation of the mutated target gene does not perform beneficial effects and may even be harmful.
Future work on this topic will aim to address challenges related to delivery and immunogenicity, increase the potential for oligonucleotide therapy to progress through clinical trials, and address the need for TSG upregulation in cancer. In addition, as targeted oligonucleotide therapies begin to enter the clinic, the use of oncogenomic sequencing could help match patients to the ideal treatment based on their tumor genetic profile.
Reference
1. Gregory, Georgina L., and Ian M. Copple. “Modulating the expression of tumour suppressor genes using activating oligonucleotide technologies as a therapeutic approach in cancer.” Molecular Therapy-Nucleic Acids (2023).
