Hemophilia is a genetic disorder characterized by the deficiency of clotting factors, resulting in spontaneous or excessive bleeding during surgery or trauma. Hemophilia B is caused by the deficiency of clotting factor IX (FIX), with an incidence of 1 in every 25,000 male infants. While several treatment approaches for hemophilia have been developed, there are still unmet needs in terms of efficacy, safety, treatment simplicity, and cost.
Recently, researchers from Seoul National University published a study titled “In vivo genome editing for hemophilia B therapy by the combination of rebalancing and therapeutic gene knockin using a viral and non-viral vector” in the journal Molecular Therapy: Nucleic Acids. The study revealed the use of viral and non-viral vectors, combined with rebalancing and therapeutic gene knockin, for in vivo genome editing in the treatment of hemophilia B.
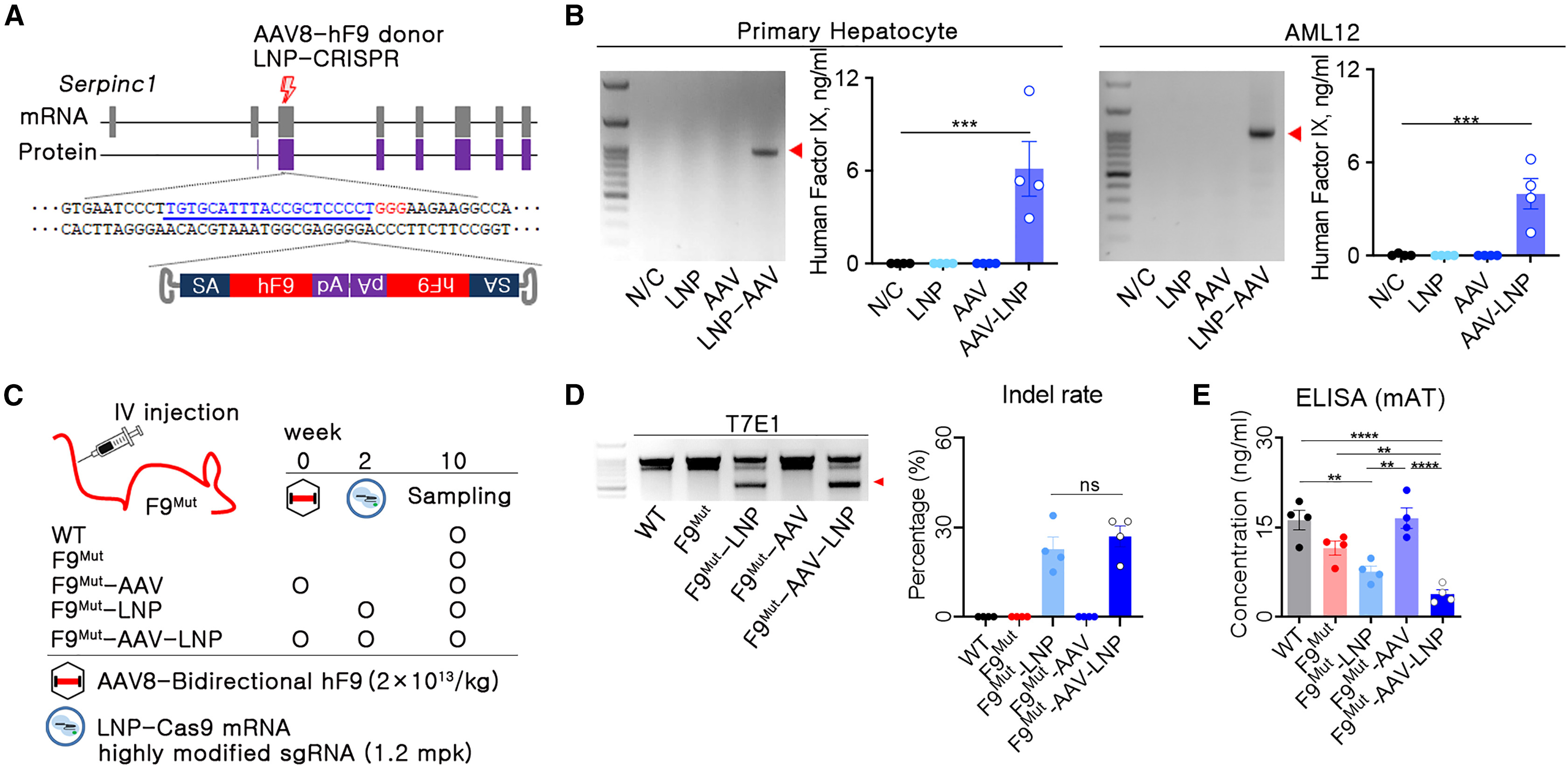
Current treatment strategies for hemophilia involve long-term therapeutic gene expression using recombinant adeno-associated virus (AAV) and rebalancing therapy by downregulating anticoagulant pathways. However, these methods have limitations in terms of immune reactions or are insufficient to control acute bleeding. Therefore, the researchers developed a strategy for treating hemophilia B by combining rebalancing with lipid nanoparticles (LNPs) and AAV-mediated human factor IX (hF9) gene knockin (KI).
Antithrombin (AT; Serpin family C member 1 [Serpinc1]) was selected as the target gene for KI to downregulate the anticoagulant pathway. First, the combined use of LNPs encapsulating clustered regularly interspaced short palindromic repeats (CRISPR) and AAV donors resulted in 20% insertions or deletions (Indels) in Serpinc1, resulting in a 67% decrease in mouse AT concentration. Secondly, the hF9 encoding sequence was integrated into approximately 3% of the target gene locus.
HF9KI produced approximately 1,000 ng/mL of human clotting factor IX (HFIX) and restored coagulation activity to normal levels. The injection of LNP-CRISPR led to sustained downregulation of AT and long-term production of hFIX for up to 63 weeks. In the case of partial hepatectomy, the capacity for AT inhibition and hFIX protein production could be maintained by the proliferation of gene-edited liver cells.
The combined administration of AAV and LNP did not result in severe adverse effects except for random integrations. This study demonstrates that the treatment of hemophilia B can be achieved through the combination of rebalancing with LNPs and AAV-mediated hF9KI.
In conclusion, this study confirms that the combination strategy of AT targeting and hF9KI restores coagulation activity. The use of AAV and LNP hybrids achieved high in vivo therapeutic gene knockin, which appeared to be sustained for up to 63 weeks. Furthermore, the AAV-LNP combination may be advantageous in reducing AAV dosage and could serve as a foundation for the development of advanced therapeutic strategies for protein deficiency-related genetic diseases based on gene editing, as compared to the dual AAV strategy.
Reference
1. Lee, Jeong Hyeon, et al. “In vivo genome editing for hemophilia B therapy by the combination of rebalancing and therapeutic gene knockin using a viral and non-viral vector.” Molecular Therapy-Nucleic Acids 32 (2023): 161-172.
