The CRISPR-Cas system, a DNA recognition tool, has rapidly advanced as a means of editing cellular DNA. In the engineered version, a single guide RNA (gRNA) guides Cas9 to the target sequence adjacent to the so-called “Protospacer Adjacent Motif” (PAM; 50-NGG-30) and then cleaves the dsDNA, which is repaired by non-homologous end joining (NHEJ).
As a result, small insertions or deletions (indels) occur at the cleavage site, which may lead to frameshift and loss of activity in affected proteins. In addition, the homology-directed repair (HDR) pathway is activated and can be utilized to insert DNA at the target site. The CRISPR-Cas system has also been used to inactivate the viral DNA of human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), hepatitis B virus (HBV), herpes virus, and human papillomavirus (HPV).
However, in most cases, viral DNA exists in a non-replicative state, either integrated into the host chromosome or as stable extrachromosomal forms with low copy numbers. In these studies, the main goal is to eliminate the viral genome from infected cells because they are resistant to conventional drug elimination.
Recently, researchers from the Krembil Research Institute at the University of Toronto published an article titled “Inhibition of adenovirus replication by CRISPR-Cas9-mediated targeting of the viral E1A gene” in the journal Molecular Therapy: Nucleic Acids. The study reveals the inhibitory effect of CRISPR-Cas9 targeting the viral E1A gene on adenovirus replication.
The CRISPR-Cas system targeting DNA can cleave dsDNA in mammalian cells. Therefore, it is used to target the genomic dsDNA of viruses, primarily when present in cells at low copy numbers in a non-replicative state. However, the ability of the CRISPR-Cas system may be limited by certain rapidly replicating viruses that produce a large amount of viral DNA in a short period of time.
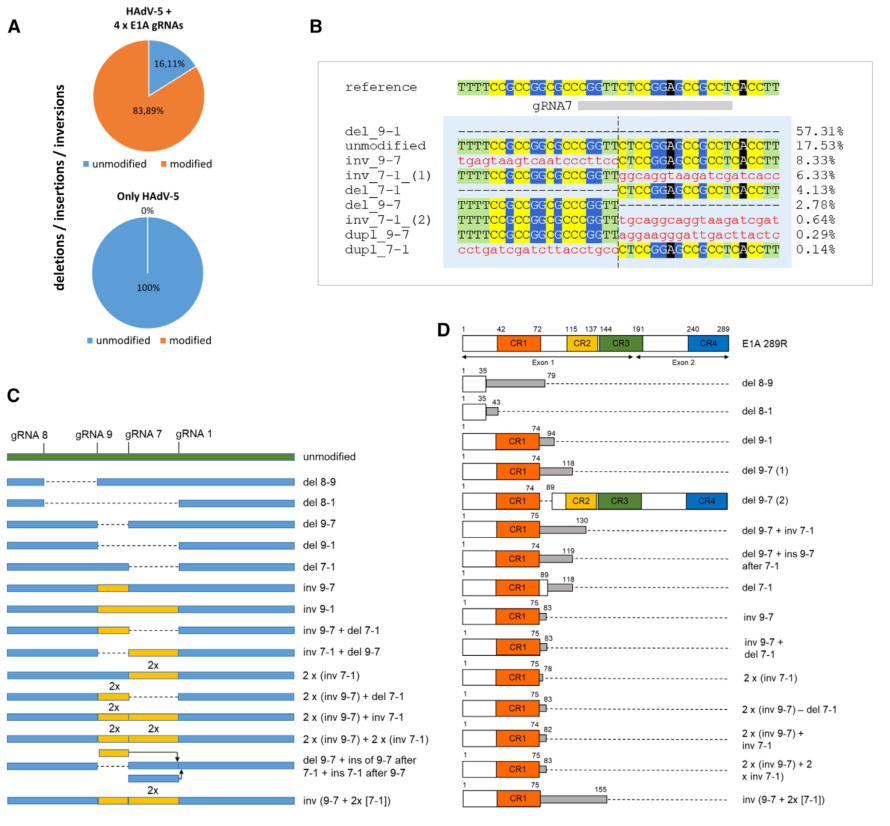
The accessibility of viral DNA replication sites, the accessibility of pre-encapsidated DNA, and its interaction with shielding proteins are further potential barriers. Adenovirus is a rapidly replicating dsDNA virus for which no approved antiviral therapy currently exists. We targeted the essential regulatory subunit E1A with a set of guide RNAs to evaluate the effectiveness of CRISPR-Cas9 in inhibiting human adenovirus 5 replication in vitro and observed a reduction of infectious viral particles by three orders of magnitude.
Furthermore, the cleavage of target DNA negatively impacted the accumulation of viral DNA during the infection cycle. This result was primarily due to specific deletions, inversions, and duplications occurring between the targets, which in most cases abolished the majority of E1A functions. Furthermore, we compared the efficacy of multiple gRNA expression strategies and obtained comparable results.
Considering the risks associated with systemic administration, localized treatment of localized infections may offer a higher chance of success. The eye presents an ideal target for gene therapy due to the ability to achieve high vector titers with small volumes and the minimal systemic distribution and side effects in this enclosed organ. Therefore, localized adenovirus infections in the eye can be targeted for localized treatment using future therapies based on CRISPR-Cas9.
Reference
1. Didara, Zrinka, et al. “Inhibition of adenovirus replication by CRISPR-Cas9-mediated targeting of the viral E1A gene.” Molecular Therapy-Nucleic Acids 32 (2023): 48-60.
