Deubiquitination Enzyme Regulates the Biosynthesis of Membrane Proteins
Many proteins with hydrophobic transmembrane domain (TMD) can transfer from cytoplasm to cell membrane after translation. However, it is not clear how hydrophobic membrane proteins escape from the recognition of cytosolic protein quality control (PQC). Previous studies have found that the quality control program can usually identify the hydrophobic structure of misfolded proteins and mark them for proteasomal degradation by adding ubiquitin chains.
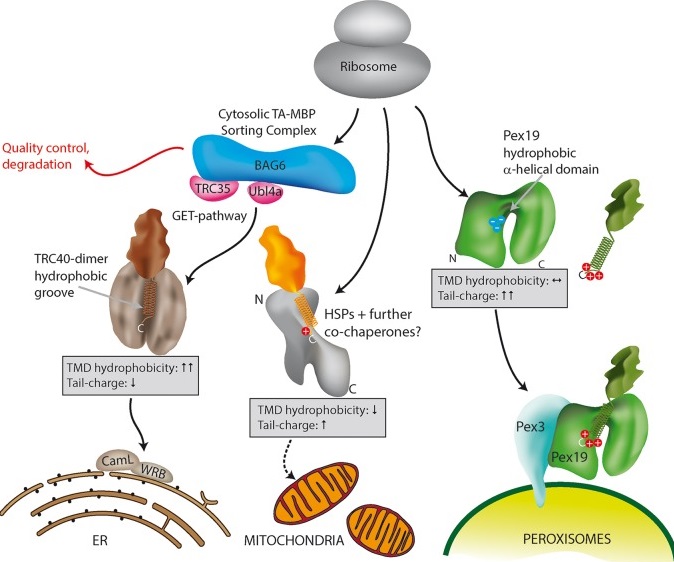
Schematic model for TA protein targeting to ER, mitochondria and peroxisomes in mammalian cells. (Journal of Cell Science)
A recently published study found that the tail-anchor (TA) protein can be recognized by cytosolic PQC and ubiquitinated immediately after synthesis in cells. Unexpectedly, the ubiquitinated TA protein did not enter the proteasome for degradation, but was transferred to the vicinity of TRC40 and delivered to endoplasmic reticulum (ER) for insertion. ER-related deubiquitination enzymes USP20 and USP33 removed TA proteins after they were inserted into ER. This result shows that deubiquitination can help membrane proteins avoid being recognized and degraded by PQC in the cytoplasm, thus transferring to the cell membrane correctly.
The study hypothesized that TA proteins should not be ubiquitinated and degraded in cells if they were not affected by the cytosolic PQC mechanism. To test this, the researchers constructed TA proteins that differ in the hydrophobicity of C-terminal TMD. First of all, the experiment examined whether the newly synthesized TA protein was ubiquitinated in the cytoplasm before targeting and inserting into the ER membrane. The results showed that the in vitro translation of TA protein Sec61β produced a modification consistent with Sec61β ubiquitination, which was consistent with the previous studies. The removal of TMD results in a significant loss of ubiquitination that only occurs in Sec61β because TMD lacks the lysine residues to be ubiquitinated.
Next, the experiment explored the details of protein ubiquitination. The results showed that the TA protein carried a K48 ubiquitination tag, indicating that its ultimate fate may enter the proteasome for degradation. Based on this, it was also found that the ubiquitination process of TA protein was not dependent on cell targeting factors, and it was further found that ubiquitinated TA protein attached to the endoplasmic reticulum and accepted the deubiquitination of USP20 and USP33 on the endoplasmic reticulum surface.
In summary, this study reveals the molecular mechanism of how proteins with hydrophobic domains are successfully transferred to the cell membrane in the cytoplasm without being degraded by ubiquitination.