Antibody-expressing Oncolytic Virus
As a first-class biotech manufacturer, Creative Biolabs has established a comprehensive oncolytic virus engineering platform. With our excellent experts, Creative Biolabs provides high quality antibody-expressing oncolytic virus to meet your project development. We are dedicated to designing and developing the desirable oncolytic virus to contribute greatly to your research, preclinical study and drug development.
Recently, recombinant monoclonal antibodies (mAbs) are one of the most powerful therapeutic classes in inflammatory diseases and oncology. However, a broader accessibility and implementation is limited by the high product cost and long-term need for frequent administration. Besides, in order to seek more effective mAb combination therapies, the costs and risk of toxicity are further increased. To solve these problems, antibody gene transfer has developed to administer to patients the mAb-encoding nucleotide sequence instead of the mAb protein. Among various antibody gene transfer methods, oncolytic virus has been proven to be a very powerful one. Generally, there are two antibody categories that are applied to arm oncolytic virus, tumor-targeting mAbs and immunomodulatory mAbs. Creative Biolabs is proud to offer customized antibody-expressing oncolytic virus to facilitate your project.
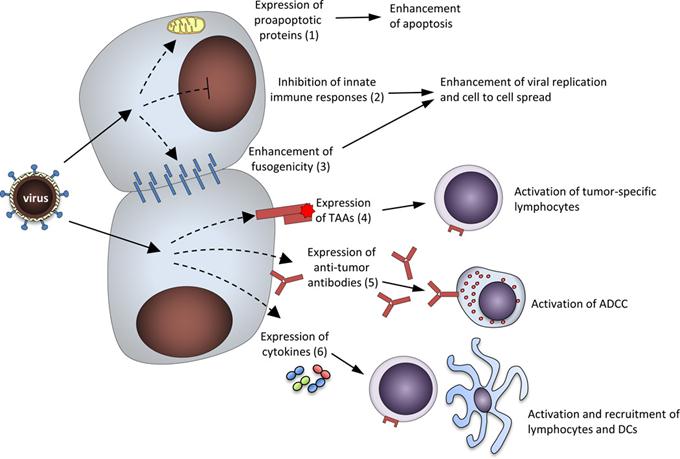 Fig 1. Approaches used to promote anti-tumor activity of NDV via genetic engineering, including expressing anti-tumor antibodies. (Davis, D., 2015)
Fig 1. Approaches used to promote anti-tumor activity of NDV via genetic engineering, including expressing anti-tumor antibodies. (Davis, D., 2015)
Tumor-targeting mAbs
Oncolytic viruses enhance anti-tumor responses via selective tumor cell killing and induction of systemic anti-tumor immunity. Tumor-targeting mAbs is the first mAbs type used to arm oncolytic viruses. Local intratumoral expression is an attractive approach to overcoming poor mAb penetration in solid tumors. For example, researchers have successfully armed replication-competent oncolytic vaccinia viruses with a scFv which directed against both human and murine vascular endothelial growth factor (VEGF). After i.v. injection, tumor-specific delivery and continued scFv production is observed in mouse human lung cancer xenograft models. Moreover, the anti-VEGF-scFv armed virus has a stronger anti-tumor response than the unarmed virus. In addition, other researchers report similar findings following intratumoral injection of an oncolytic adenovirus armed with full length anti-HER2 trastuzumab.
Immunomodulatory mAbs
Another more relevant type to arm oncolytic viruses is immunomodulatory mAbs. Recently, a variety of Phase I trials are underway to determine the combination of oncolytic viruses and conventional immunomodulatory mAb administration. However, systemic treatment with checkpoint-blocking mAbs enables to induce severe immune-related adverse effects, highlighting the opportunity for local therapies, such as via mAb-armed oncolytic viruses. Researchers have armed a replication-deficient and -competent oncolytic adenovirus with an anti-human CTLA-4 mAb. After intratumoral delivery in nude mice xenograft models, the armed replication-competent virus exhibits an increased anti-tumor effect compared to the unarmed virus, despite the lack of immunological function the anti-human CTLA-4 mAb had in these mice. Besides, after intratumoral injection of the armed replication-competent oncolytic virus, the mAb levels in tumors and plasma are remarkably higher than the replication-deficient armed virus. What's more, another research has demonstrated that i.v. injection of a replicating adenovirus expressing an anti-murine CTLA-4 mAb delayed tumor growth in syngeneic mouse models, and resulting in complete regressions when combined with a virus encoding GM-CSF.
Other Services in Oncolytic Virus Engineering Platform
- Pathogenicity Manipulation (Attenuation)
- Immunogenicity Manipulation
- Cytokine/Chemokine-expressing Oncolytic Virus
- Immune Checkpoint Inhibitor-expressing Oncolytic Virus
With our well-established oncolytic virus engineering platform, the experienced scientists at Creative Biolabs are dedicated to helping you developing unique oncolytic virus. We also provide other various services regarding oncolytic virus development. Please feel free to contact us for more information and a detailed quote.
Reference:
- Hollevoet, K., (2017). "State of play and clinical prospects of antibody gene transfer." Journal of translational medicine, 15(1), 131.
- Freedman, J. D., (2017). "Oncolytic adenovirus expressing bispecific antibody targets T‐cell cytotoxicity in cancer biopsies." EMBO molecular medicine, 9(8), 1067-1087.
- Davis, D., (2015). Application of Oncolytic Viruses for Cure of Colorectal Cancer.
