Immune Checkpoint Inhibitor-expressing Oncolytic Virus Production Service
Introduction
Oncolytic virotherapy and immune checkpoint inhibitor therapy are promising frontier technologies for tumor therapy. Oncolytic viruses are genetically modified to replicate in tumor cells and kill tumor cells without affecting normal cellular activities. Immune checkpoint inhibitors by blocking immune checkpoint molecules, relieve inhibition of tumor cells of the immune system, to activate the immune cells of tumor cell killing ability. Through gene editing, oncolytic viruses can express genes related to immune checkpoint inhibitors, which is a potential novel tumor therapy strategy.
Based on the mature oncolytic virus engineering platform, Creative Biolabs provides a full range of customized solutions according to client needs to produce high-quality oncolytic virus products expressing immune checkpoint inhibitors.
Oncolytic Viruses with Anti-PD-1 Therapy
Programmed cell death-1 (PD-1) is a transmembrane glycoprotein expressed on activated T cells, B cells, and NK cells. Programmed cell death ligand 1 (PD-L1) is normally expressed on the surface of antigen-presenting cells (APC) and tumor cells. PD-1 is activated by inflammatory stimuli and interferes with the immune system, leading to cancer cell development. PD-L1 is expressed at high levels after induction by cytokines (such as IFN-γ and TNF-α) in the tumor microenvironment. PD-1 combines with PD-L1, inhibits the activation of T cells, induces T cell apoptosis, reduces the secretion of cytokines, and helps tumors escape immune surveillance. Researchers arm oncolytic influenza virus with transgenes that encode immune checkpoint inhibitor αPD-L1, effectively reducing the expression level of PD-L1 on HCC cells, and activating CD8+T cells through the cGas-STING signaling pathway1. Veinalde, et al. encode monoclonal antibody drugs of αPD-1 or αPD-L1 (pembrolizumab and atezolizumab, etc.) into MVS and improve antitumor efficacy in preclinical mouse tumor models2.
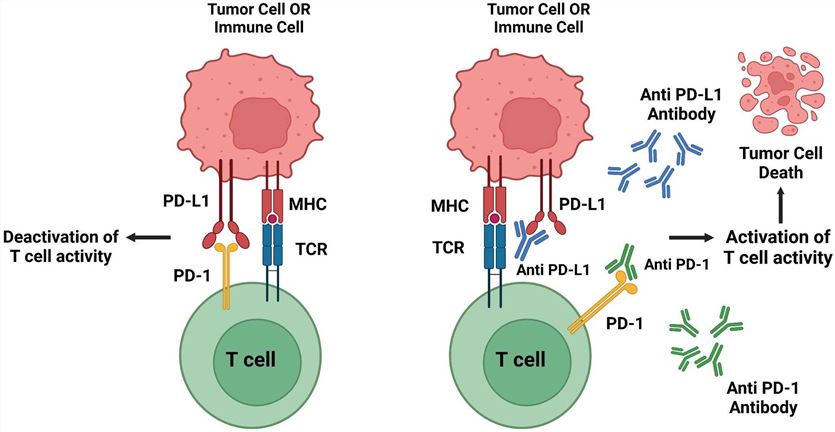 Fig.1 Schematic representation of the mechanism of action of PD-1/PD-L13,6
Fig.1 Schematic representation of the mechanism of action of PD-1/PD-L13,6
Oncolytic Viruses with Anti-CTLA-4 Therapy
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a member of the immunoglobulin superfamily (IgSF). The binding of CTLA-4 to ligand B7-2 protein inhibits the proliferation and activation of T cells and cytokine production while reducing immune responses. Inhibition of CTLA-4 reactivates T cells and restores their ability to attack cancer cells. However, CTLA-4 inhibitors do not work against all types of cancer in clinical practice.
Researchers construct an attenuated MV vector encoding CTLA-4 antibody (MV-αCTLA-4) and characterize it in a mouse model of melanoma. The results show that antibody-encoded MV can significantly delay tumor progression, increase T cell numbers, and enhance anti-tumor immunity.
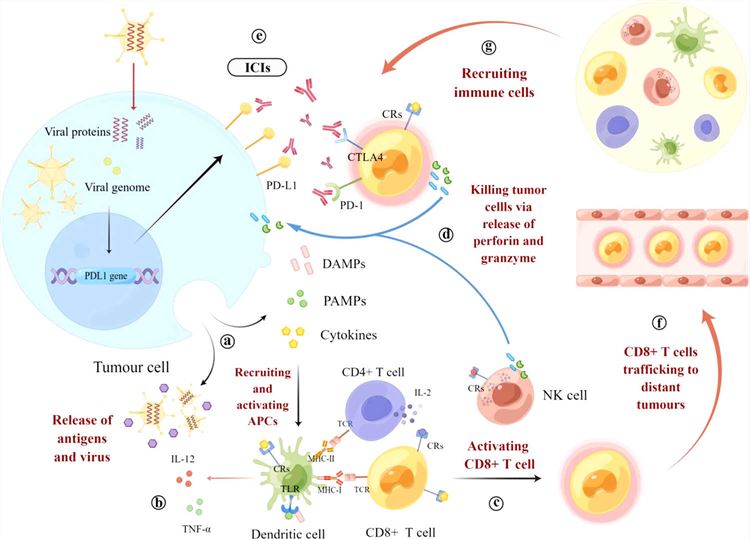 Fig.2 Mechanism of OVs combined with ICIs to stimulate anti-tumor immunity4,6
Fig.2 Mechanism of OVs combined with ICIs to stimulate anti-tumor immunity4,6
Oncolytic Viruses with Anti-TIGIT Therapy
T cell immunoglobulin and ITIM domain (TIGIT) is a cell surface protein with immunosuppressive function, which is mainly expressed on T cells and NK cells. In general, TIGIT inhibits T cell immune activity and induces NK cell exhaustion, thereby inhibiting NK cell killing activity. Researchers find that tumor cells infected with VV encoding TIGIT antibodies produce and secrete functional αTIGIT, which increases the infiltration of CD8+ T cells around the tumor, generates long-term immune memory, and enhances the anti-tumor effect of VV.
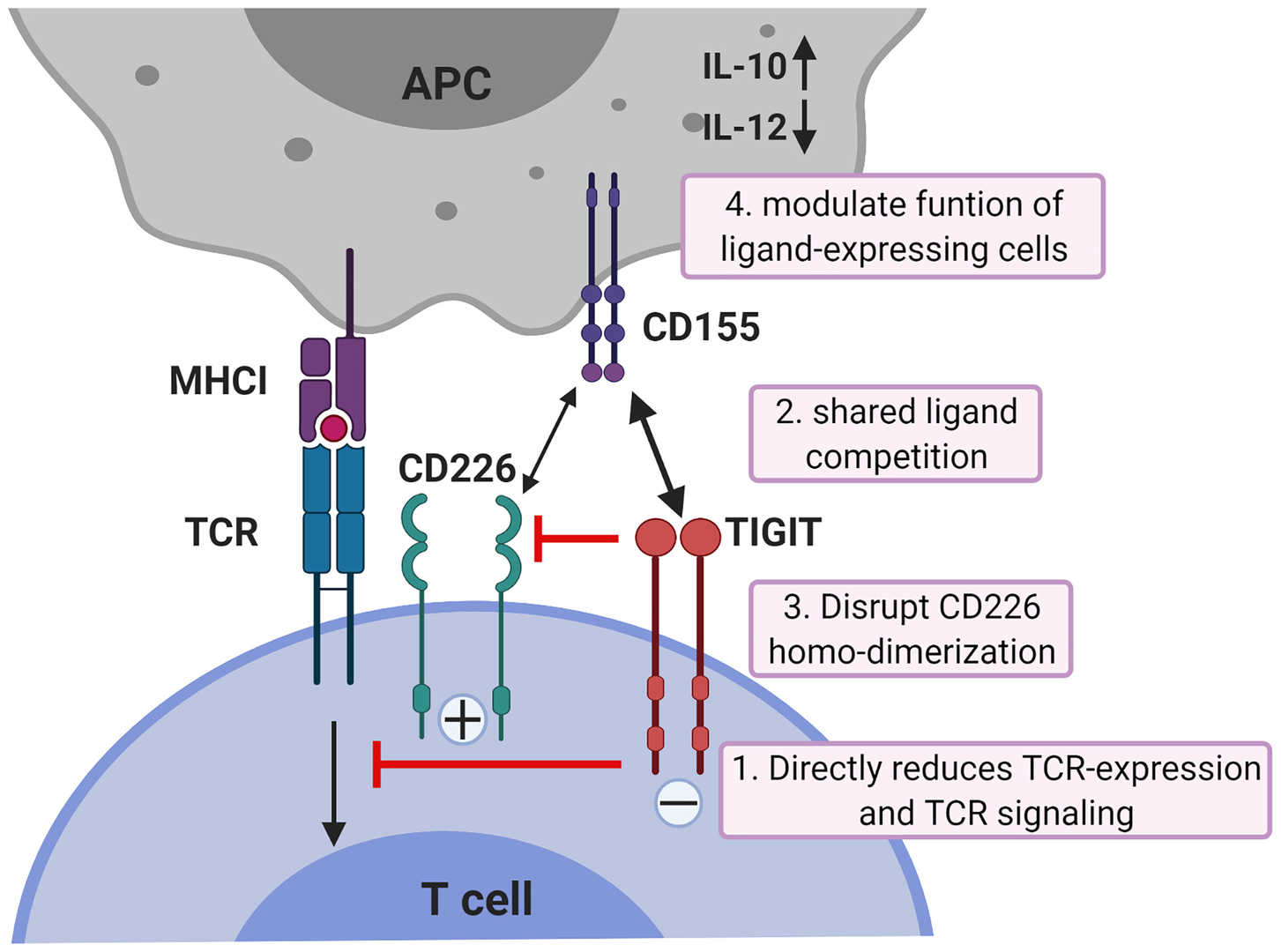 Fig.3 Mechanisms of TIGIT inhibition in T cells5,6
Fig.3 Mechanisms of TIGIT inhibition in T cells5,6
Other Services on Oncolytic Virus Engineering Platform
- Pathogenicity Manipulation (Attenuation)
- Immunogenicity Manipulation
- Antibody-expressing Oncolytic Virus
- Cytokine/Chemokine-expressing Oncolytic Virus
Based on our well-established OVs engineering platform, the experienced scientists here at Creative Biolabs are dedicated to helping you develop a unique oncolytic virus. Please feel free to contact us for more information and a detailed quote.
References
- Sun, Fang, et al. "A recombinant oncolytic influenza virus expressing a PD-L1 antibody induces CD8+ T-cell activation via the cGas-STING pathway in mice with hepatocellular carcinoma." International Immunopharmacology 120 (2023): 110323.
- Veinalde, Rūta, et al. "Oncolytic measles vaccines encoding PD-1 and PD-L1 checkpoint blocking antibodies to increase tumor-specific T cell memory." Molecular Therapy-Oncolytics 24 (2022): 43-58.
- Parvez, Adil, et al. "PD-1 and PD-L1: architects of immune symphony and immunotherapy breakthroughs in cancer treatment." Frontiers in Immunology 14 (2023): 1296341.
- Ren, Yi, et al. "Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option." Frontiers in immunology 13 (2022): 961796.
- Ge, Zhouhong, et al. "TIGIT, the next step towards successful combination immune checkpoint therapy in cancer." Frontiers in immunology 12 (2021): 699895.
- Distributed Under Open Access license CC BY 4.0, without modification.
