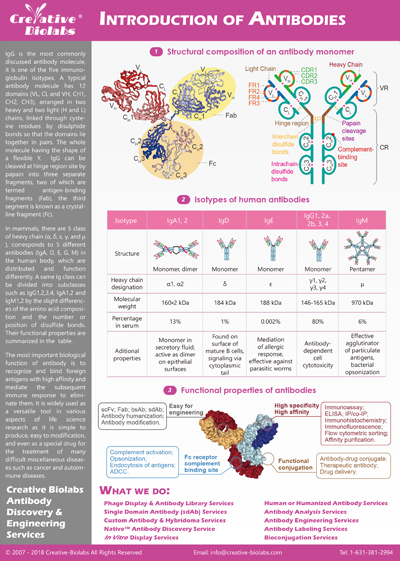
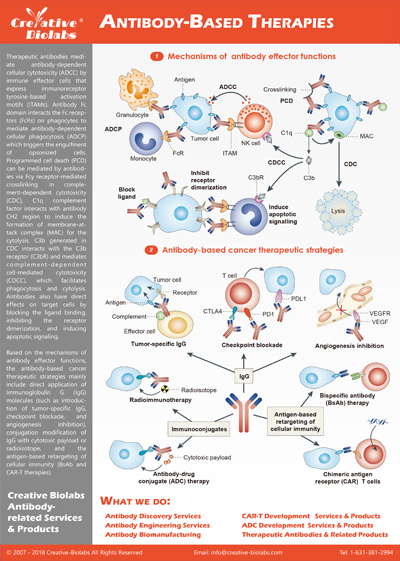
Antibody-drug conjugates (ADCs) are novel targeted cancer treatments that combine the specificity of monoclonal antibodies with powerful cytotoxic drugs, delivering themselves to cancer cells without damaging healthy tissue. ADCs exploit the antibody's
ability to go after tumor antigens, delivering cytotoxic drugs with accuracy, efficacy, and fewer side effects than traditional chemotherapy. The personalized strategy has yielded several approved ADCs, including brentuximab
vedotin for Hodgkin's lymphoma and trastuzumab emtansine for HER2-positive breast cancer, with scores more on the drawing board. Creative Biolabs is the ADC research pioneer, offering comprehensive ADC solutions across
the ADC development lifecycle, from antibody engineering to linker chemistry to cytotoxic payload optimization, to drive next-generation cancer therapies.
Talk to an Expert →


-Technology-1.png)
-Technology-2.png) Fig.2 General structure of antibody-drug conjugate.2
Fig.2 General structure of antibody-drug conjugate.2