Why MEA for iPSC Models?
The advent of iPSC technology has transformed the landscape of biomedical research. By reprogramming somatic cells, such as skin or blood cells, back to a pluripotent state, we can generate a virtually limitless supply of patient-specific cells. These iPSCs can then be differentiated into any cell type in the human body, including those that were previously difficult to source, such as cardiomyocytes and neurons.
MEA is a non-invasive technique that allows for the recording of extracellular field potentials from electrically active cells, such as neurons and cardiomyocytes. When iPSC-derived cells are cultured on these plates, they form a functional network, and the electrodes detect the electrical signals generated by the cells as they communicate with each other. This provides a wealth of information about the electrophysiological properties of the cells, including:
- Action Potential Firing: The fundamental unit of electrical signaling in excitable cells.
- Field Potential Duration: An important parameter in cardiomyocytes that corresponds to the QT interval in an electrocardiogram (ECG).
- Spike Train Analysis: The pattern of action potential firing in neurons, which can reveal information about neuronal activity and network connectivity.
- Network Bursting: Synchronized firing of a population of neurons, which is a hallmark of neuronal network activity.
- Conduction Velocity: The speed at which an electrical signal propagates across a network of cells.
The ability to measure these parameters in a high-throughput format makes MEA an ideal platform for screening compounds for their effects on cellular electrophysiology.
Sample Types We Support
- iPSC-derived neurons: excitatory glutamatergic, GABAergic inhibitory, dopaminergic, sensory, motor neurons.
- Glial co-cultures: astrocytes, microglia for neuroinflammation and homeostasis models.
- Human iPSC cardiomyocytes: ventricular-like, atrial-like, and mixed phenotypes; cryo-thawed kits or in-house differentiated lines.
- 3D aggregates & organoids: neurospheroids or cardiac microtissues conditioned for MEA attachments.
- Custom formats: client-provided lines, novel differentiation protocols, and disease-specific isogenic panels.
Not sure whether your model fits MEA? Share your cell identity markers, plating density, and maturation schedule—we will align the plate format, coating, and recording cadence to your biology.
Platforms, Formats & Environments
| Platforms, Formats & Environments | Description |
|---|---|
| Planar MEA (24/48/96-wells) | For scalable pharmacology and multi-condition runs. |
| High-density MEA (HD-MEA) | For fine-grained propagation mapping and micro-network resolution. |
| Environmental Control | 37 °C, 5% CO₂, humidity-stable lids, perfusion-compatible for long recordings. |
| Stimulation Capabilities | Electrical pacing (cardiac), network stimulation (neuronal), and optogenetic triggers where applicable. |
| Coating Chemistries | PEI/PLO/laminin, fibronectin, Matrigel alternatives—selected for your cell type and endpoint. |
Service Modules We Provide
Creative Biolabs offers a complete end-to-end solution for your iPSC MEA electrophysiology needs. Our services are fully customizable to meet the specific requirements of your project.
- iPSC Generation and Differentiation - We can generate high-quality iPSCs from various samples or provide you with our well-characterized, control iPSC lines. We have optimized protocols for the differentiation of iPSCs into a wide variety of cell types, including cardiomyocytes, cortical neurons, dopaminergic neurons, motor neurons, and sensory neurons.
- MEA Assay Development and Validation - We have a team of experienced electrophysiologists who can develop and validate custom MEA assays to meet your specific research needs. We can optimize cell seeding densities, culture conditions, and recording parameters to ensure the highest quality data.
- High-Throughput Screening - Our state-of-the-art MEA platforms allow for the high-throughput screening of compound libraries for their effects on cellular electrophysiology.
- Advanced Data Analysis - We have developed a sophisticated data analysis pipeline that allows for the extraction of a wide range of electrophysiological parameters from MEA recordings. We can provide you with a comprehensive data analysis report, including statistical analysis and graphical representation of the results.
Wide Applications of iPSC Technology and MEA
The combination of iPSC technology and MEA creates a powerful synergy, providing a highly predictive in vitro platform for a wide range of applications. By using human iPSC-derived cells on MEA, we can assess the functional consequences of genetic mutations, disease states, and drug exposure in a human-relevant context.
Our iPSC MEA electrophysiology characterization services are particularly valuable for:
| Applications | Descriptions |
|---|---|
| Cardiotoxicity Assessment | Our iPSC-derived cardiomyocyte MEA assays provide a highly sensitive and predictive platform for assessing the proarrhythmic potential of new chemical entities. We can measure key parameters, providing a comprehensive assessment of cardiac risk. |
| Neurotoxicity Assessment | Our iPSC-derived neuron MEA assays can be used to assess the effects of compounds on neuronal firing, network activity, and synaptic function. This allows for the early identification of compounds with the potential to cause neurotoxic side effects, such as seizures or neuronal cell death. |
| Phenotypic Screening | Our iPSC MEA platform can be used to screen compound libraries for their ability to modulate the electrophysiological phenotype of disease-relevant cell types. |
| Mechanism of Action Studies | MEA can be used to investigate the mechanism of action of lead compounds by assessing their effects on specific electrophysiological parameters. This can provide valuable insights into how a compound works and help to guide lead optimization efforts. |
| Phenotype Characterization | We can use MEA to characterize the electrophysiological phenotype of iPSC-derived cells from patients with a wide range of diseases. |
| Genotype-Phenotype Correlations | By comparing the electrophysiological properties of iPSC-derived cells from patients with different genetic mutations, we can establish genotype-phenotype correlations and gain a better understanding of the molecular basis of disease. |
Published Data
In this study, to test whether the differentiated cardiomyocytes had proper electrophysiological properties, researchers employed MEA, and challenge them with different cardiac drugs. MEA records electrical waveform signals that are called extracellular field potentials (FPs) and which are generated and shaped by monolayers or small clusters of cardiomyocytes. FP contour represents the cardiac action potential and reflects to some extent the electrocardiogram recording.
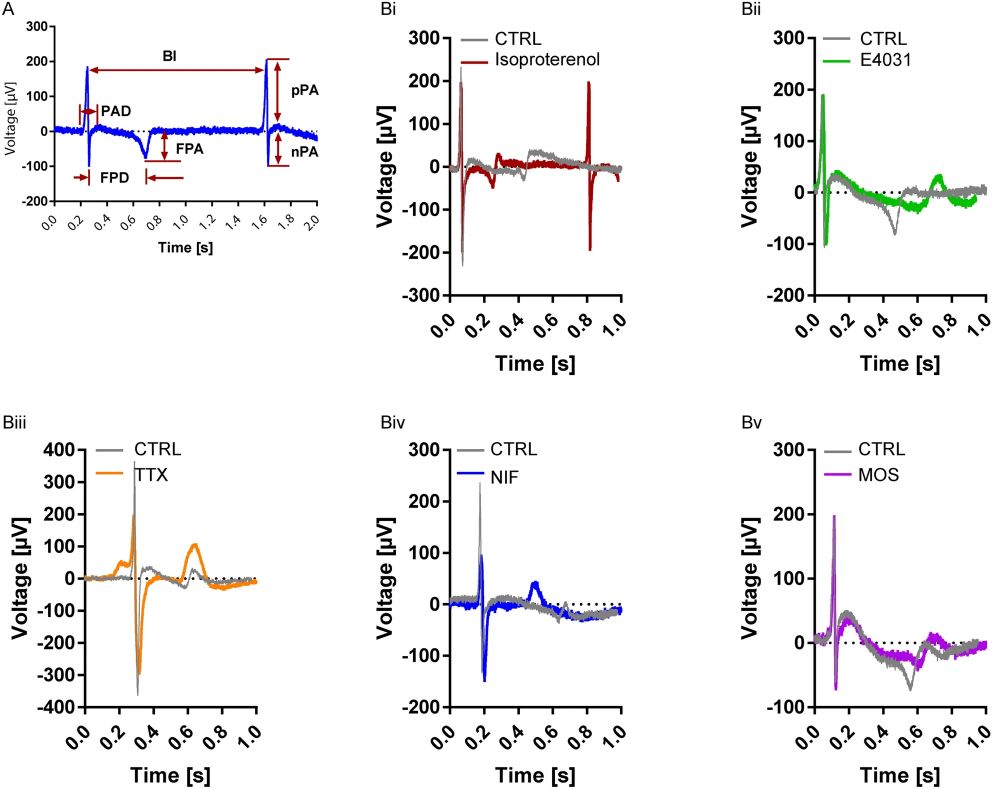 Fig. 1 MEA recording of hiPSC-derived cardiomyocytes.1,3
Fig. 1 MEA recording of hiPSC-derived cardiomyocytes.1,3
One high-throughput in vitro functional evaluation method that uses cultured neurons involves performing measurements with a planar MEA. Use of an MEA is a noninvasive method of measuring neural network activity at multiple points simultaneously. With modern advancements in multi-well testing, the effects that compounds have on nerve function can be measured in a high-throughput fashion. Previous studies have used rodent neurons to detect seizure-causing compound response.
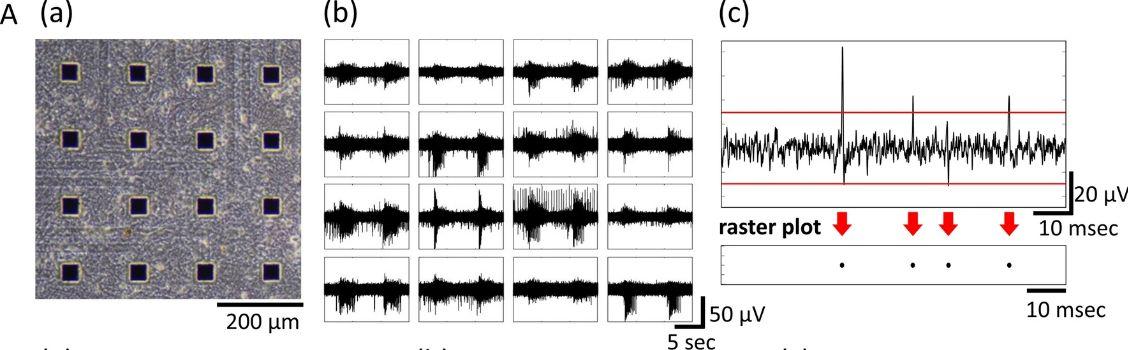 Fig. 2 MEA data from a cultured human iPSC-derived neural network.2,3
Fig. 2 MEA data from a cultured human iPSC-derived neural network.2,3
What Our Clients Say
"We were investigating a series of compounds for potential neurotoxicity, and the iPSC-derived neuron MEA assay provided clear, actionable insights. The team's deep expertise was evident from the initial project discussion through to the final report."
— Dr. Alistair Finch, Senior Director of Preclinical Safety
"Creative Biolabs didn't just run a standard assay, they worked with us to customize the protocol to answer our specific research questions. The quality and reproducibility of their iPSC-cardiomyocyte MEA data were instrumental for our recent publication in a high-impact journal."
— Dr. Elena Petrova, Principal Investigator
"Partnering with Creative Biolabs gave us access to state-of-the-art technology and the scientific team to run it effectively. The project management was seamless, and the team was incredibly responsive. We absolutely plan to work with them again for our future programs."
— Dr. Jian Li, Chief Scientific Officer
FAQs
Q: Can your MEA assay protocols be customized?
A: One of our core principles is to engage in scientific collaboration with clients, delivering highly customized testing solutions. Our team of scientists will work closely with you to design and optimize experimental protocols best suited to your research objectives. Customizable parameters include, but are not limited to cell models, drug treatment regimens, assay endpoints and data analysis, and combination testing. Prior to project initiation, we will conduct in-depth technical discussions with you to ensure the final experimental design precisely addresses your scientific questions.
Q: What MEA platforms and plate formats do you use?
A: We operate MEA systems and support 24-, 48-, 96- and high-density formats. Platform selection balances throughput, sensitivity, and your assay goals. We align plate choice with expected signal strength, plating density, and required replicates to ensure statistical power and cost-efficiency.
Q: How mature do cultures need to be before recording?
A: For neuronal assays, we typically record baseline activity after spontaneous network formation, then track weekly as networks stabilize. Exact timing depends on lineage, plating density, and co-culture composition.
Q: Do you also support iPSC-cardiomyocyte MEA?
A: Yes. For cardiomyocytes, we quantify beat rate/regularity, field potential duration (FPD), conduction velocity, and arrhythmia indices. Reference controls often include isoproterenol and E-4031.
Q: What about pricing and typical timelines?
A: Pricing depends on model complexity, plate format, number of conditions/doses, and duration of longitudinal recording. We provide a transparent quote and a Gantt-style schedule aligned to your internal milestones.
Partner with the Experts in iPSC MEA Electrophysiology














1. Contact Us
via the Inquiry Form or Email
2. Define Your Needs
Cell Type, Function, Quantity, Modifications
3. Kickstart the Project
Our Expert Team Guiding Every Step
At Creative Biolabs, we are passionate about helping our clients to accelerate their research and development programs. Our iPSC MEA electrophysiology characterization services provide a powerful and predictive platform for assessing drug efficacy and safety.
Contact us today to discuss your project with one of our experts and learn how we can help you achieve your research goals.
References
- Balafkan, Novin, et al. "A method for differentiating human induced pluripotent stem cells toward functional cardiomyocytes in 96-well microplates." Scientific reports 10.1 (2020): 18498. https://doi.org/10.1038/s41598-020-73656-2
- Matsuda, N., et al. "Raster plots machine learning to predict the seizure liability of drugs and to identify drugs." Scientific reports 12.1 (2022): 2281. https://doi.org/10.1038/s41598-022-05697-8
- Distributed under Open Access license CC BY 4.0, without modification.
Created October 2025

