iPSCs (induced pluripotent stem cells) are a class of pluripotent stem cells that are derived from diverse somatic cell types. They have the capacity to differentiate into any type of cell in the body with high similarity to embryonic stem cells (ESCs). Given their easily accessible somatic cell origin and ability of robust proliferation and differentiation in vitro, iPSCs have been used for disease modeling, toxicity testing, drug screening, and transplantation therapies.
iPSCs are typically derived by introducing certain genes and factors such as Oct3/4, Sox2, Klf4, and c-Myc into a somatic cell type. These cells exhibit pluripotent properties and can differentiate into many cell types. In 2006, iPSC technology was firstly proposed by Shinya Yamanaka's team who reprogrammed mouse skin fibroblasts into iPSCs by introducing four specific genes (Myc, Oct3/4, Sox2, and Klf4) encoding transcription factors. Later, Yamanaka's group successfully converted human fibroblasts into iPSCs with the same four key genes in mouse reprogramming and using a retroviral system. Furthermore, several cell types have been also identified for iPSC production including human keratinocytes, peripheral blood cells, and renal epithelial cells in the urine.
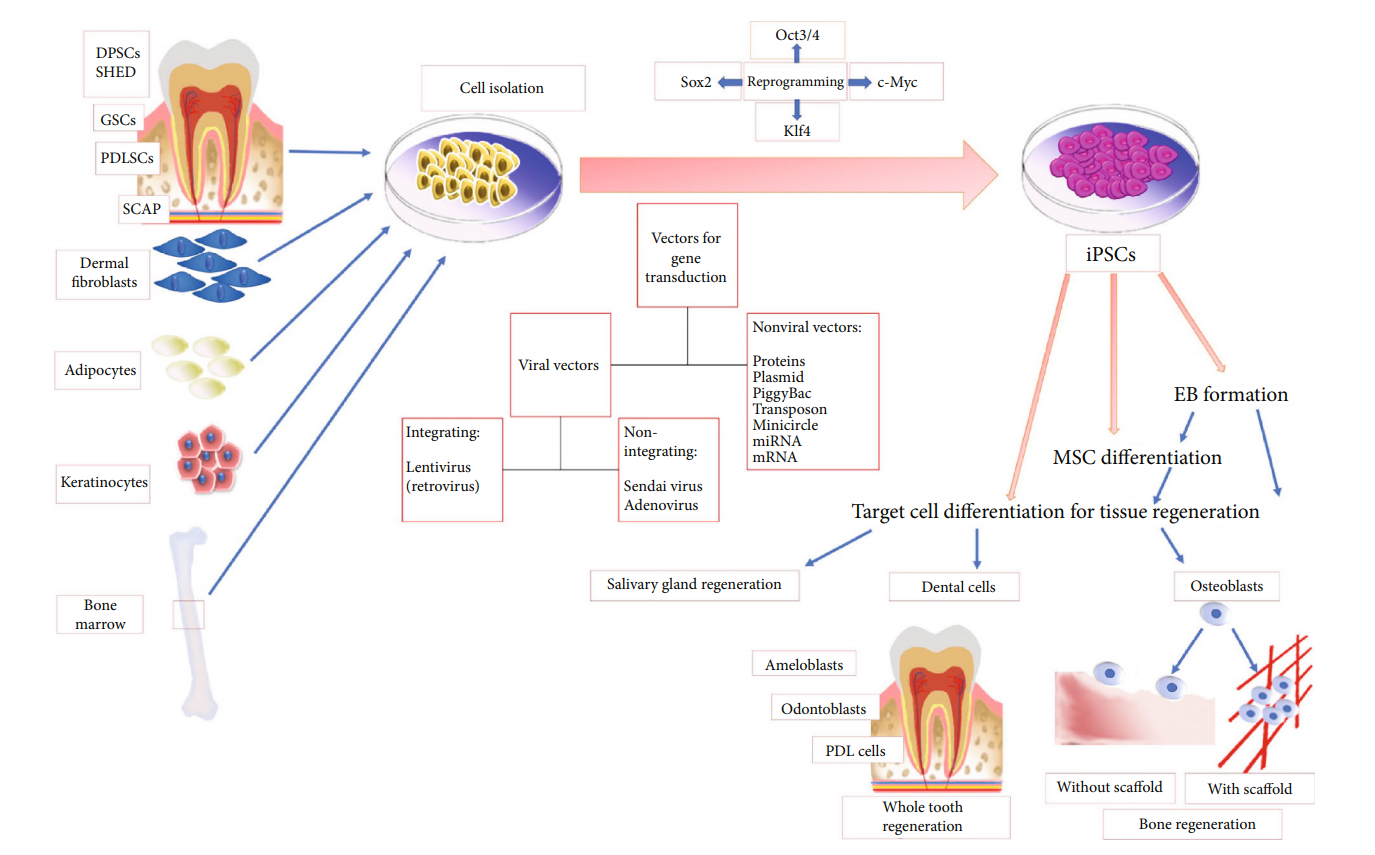 Fig.1 Diagram summarizing iPSC source, methods of gene transduction, and iPSC differentiation.1
Fig.1 Diagram summarizing iPSC source, methods of gene transduction, and iPSC differentiation.1
Factors for iPSCs generation have been identified including Oct-3/4, Sox gene family, Klf family, Myc family, Nanog, and LIN28. Genes used for the induction can be effectively introduced and integrated into the genome of the somatic cells via a retroviral vector. However, retroviral delivery may increase the risk of virus-induced tumor formation. To overcome this issue, researchers have attempted to use the transposon system to effectively deliver the key transcription factors.
Table.1 Genes used to produce iPSCs.
| Genes | Description |
|---|---|
| Oct-3/4 | Oct-3/4, a member of the family of octamer ("Oct") transcription factors, exerts a key role in maintaining pluripotency. |
| Sox family | The Sox family of transcription factors is associated with maintaining pluripotency. Sox1, Sox2, Sox3, Sox15, and Sox18 genes can induce the production of iPSCs. |
| Klf family | Klf1, Klf2, Klf4, and Klf5 were found to be factors capable of generating iPSCs. |
| Myc family | N-myc and L-myc have been identified to induce iPSCs instead of c-myc with similar efficiency. |
| Nanog | Nanog, along with Oct-3/4 and Sox2, is required for promoting pluripotency. |
| LIN28 | LIN28 is an mRNA binding protein that LIN28 is an mRNA binding protein in combination with OCT4, SOX2, and Nanog. |
| Glis1 | Glis1 is a transcription factor that has been used to induce pluripotency in combination with Oct-3/4, Sox2, and Klf4. |
Currently, most technologies have been established for iPSC research. Please click on the related text in the image below for more information.
Fig.2 Technologies for iPSCs research.
Disease modeling and drug development
iPSCs provide an unprecedented opportunity for the development of new models of human disease and drug discovery. Human patient-derived iPSCs have been shown to faithfully recapitulate hallmarks of diseased cells and tissues as well as provide unlimited materials for the discovery of new drugs. Over the past decades, the cells differentiated from patient-specific iPSCs were used to demonstrate cell-level phenotypes in monogenic diseases. However, the cell models derived from patient-specific iPSCs are not enough to demonstrate the complex phenotypes of diseases since human disease occurs within the context of complex multicellular ecosystems in which interactions occur between cells, extracellular matrices, tissues, organ systems, and pathogens. Therefore, it is required to develop more complex 3D multicellular systems such as organoids and human-rodent chimaeras. Patient-derived iPSCs have also used to construct these 3D multicellular models which more accurately recapitulate the pathobiology of a wide range of human diseases including infectious diseases, genetic disorders and cancer. Then, these diseased 3D models can be used for drug screening and validation studies. Currently, some drug candidates identified in iPSC-based systems have been under study in human trials. The application of iPSC-based disease organoid models improve preclinical drug screening and validation and accelerate candidate therapies into clinical trials. Besides, iPSC-based disease organoids models also provide powerful platforms for the discovery of personalized medicine.
The ability of iPSCs to convert differentiated somatic cells into multipotent stem cells which can produce all cell types of adult tissues, offers great potential for regenerative medicine, especially for replacing diseased or injured cells in target organs. iPSCs or iPSC-derived progenitors can be delivered into internal organs either through intravenous injection of cells or local administration of the cells via catheter placement or following open surgery. Recently, studies have reported delivering iPSCs and therapeutic cells in injectable and implantable biomaterial scaffolds could significantly increase their viability during the injection process. Besides, some strategies should be done to increase the survival of iPSC-derived cells upon transplantation. For example, codelivery of growth factors, extracellular matrix components, and supporting cells could improve the survival and optimal function of iPSC-derived cell types. Besides, using functionalization of the delivery scaffold with appropriate cell-signaling ligands can simulate the essential functions of niche cells.
iPSCs also can be used to generate human organs for transplantation. Human 'liver buds' (iPSC-LBs) have been established from a mixture of three different kinds of stem cells: hepatocytes coaxed from iPSCs; endothelial stem cells from umbilical cord blood; and mesenchymal stem cells. The liver buds were transplanted into mice, and then the 'liver' performs normal liver functions including metabolizing drugs and producing liver-specific proteins. Furthermore, iPSCs have been used to generate other tissues such as bone tissue and cardiac muscle.
With outstanding expertise in iPSC directed reprogramming and advance technologies, Creative Biolabs offers a full range of iPSCs-related services and products to accelerate the clinical application of iPSCs in disease models and drug screening as well as regenerative medicine. If you are interested in our iPSC-related services, please contact us for more details.
Reference
For Research Use Only. Not For Clinical Use.