Intracellular proteins can be degraded, processed, and presented on the cell surface in complex with MHC molecules (HLA), which can be specifically recognized by TCR (Figure 1). Antibodies that recognize pMHC like TCR are called TCR mimic antibodies (TCRm). Currently, there are research teams that have successfully developed high-affinity TCRm antibodies targeting multiple tumor targets presented by different HLA alleles. The antitumor characteristics of TCRm antibodies in preclinical models have been confirmed. However, their therapeutic effect is not very satisfactory. Therefore, considering the strategy of using TCRm to construct ADC may improve its clinical effect. In addition, it has been proven by teams that TCR mimic antibody-drug conjugates (TCRm-ADCS) exhibit good antitumor effects in vitro and in vivo.
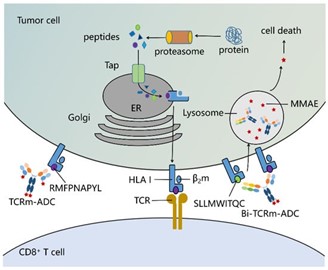 Figure 1. Peptide/human leukocyte antigen class I (HLA I) I complex as the targets of T cell receptor mimic antibody-drug conjugates (TCRm-ADCs) and bispecific(Bi)-TCRm-ADCs on the tumor cell surface (Shen Y, 2019)
Figure 1. Peptide/human leukocyte antigen class I (HLA I) I complex as the targets of T cell receptor mimic antibody-drug conjugates (TCRm-ADCs) and bispecific(Bi)-TCRm-ADCs on the tumor cell surface (Shen Y, 2019)
The WT1 cancer protein is an intracellular oncogenic transcription factor hardly expressed in normal adult tissues but overexpressed in various leukemias and solid cancers. The WT1-derived HLA-A*02:01 T-cell epitope RMFPNAPYL (RMF) is a verified target through T-cell immunotherapy.
Here, two TCRm-ADCs (ESK-MMAE and Q2L-MMAE) were generated against the WT1 RMF/HLA-A*02:01 complex, which have different affinities and can mediate specific anti-tumor activity. Although ESK-MMAE showed a higher tumor growth inhibition rate in vivo, its efficacy is not that promising, which might be due to the low expression of pHLA targets. Therefore, the bispecific TCRm-ADC was also investigated, which has a more effective tumor cytotoxicity compared to the single TCRm-ADC. In conclusion, the results confirm the feasibility of using intracellular peptides as ADC targets, expanding the range of antigen choices for antibody drugs and providing a new strategy for precise treatment of tumors.
Preparation and characterization of TCRm antibodies
Firstly, ESK and Q2L antibodies, which target the HLA-A*02:01 restricted WT1 RMF epitope, were separately expressed in 293F and 293T cells. They were subsequently purified by Protein A antibody affinity chromatography and their affinities were evaluated. The results revealed that the association constant (Ka) and dissociation constant (Kd) of ESK were higher than those of Q2L, indicating that the affinity of ESK (3.50 nM) outperforms Q2L. T2 cells are a cell line that are HLA-A0201 positive and TAP defective, lacking pMHC on their surfaces. Following exogenous addition of β2 microglobulin (β2m) and peptides, specific pMHC can form on the surface of T2 cells. K562-A2-WT1 126-134 is a genetically engineered cell line expressing HLA-A*02:01 and WT1, with WT1 RMF/HLA-A*02:01 complexes quantity on its surface being 2.4 x 103 copies per cell, whereas A431 is a HLA-A*02:01 and WT1 negative cell line, serving as the control.
Specific Srt A-mediated TCRm-ADC and its characterization
After site-specific conjugation mediated by sortase A, ESK-MMAE and Q2L-MMAE were purified by Protein A antibody affinity chromatography. The drug-antibody ratios (DAR) of ESK-MMAE and Q2L-MMAE were determined by reversed-phase high performance liquid chromatography (RP-HPLC), calculated based on the peak area ratio (DAR=((A L1 / (A L0 + A L1) + A H1 / (A H0 + A H1)) × 2). Interestingly, the DARs of both ESK-MMAE and Q2L-MMAE were 3.0. TCRm-ADC was able to bind with K562-A2-WT1 126-134 in a dose-dependent manner, implying that the higher the concentration, the more TCRm-ADC binds with the cell surface. Flow cytometry observed a slight reduction in the affinity of TCRm-ADC after conjugation.
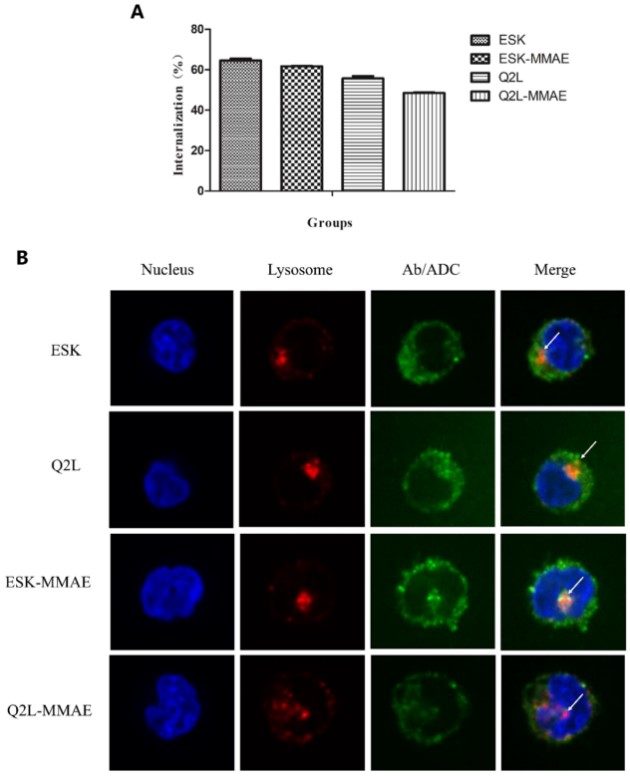
Internalization of TCRm antibody and its conjugate
Internalization is the primary method through which ADCs kill cancer cells following antigen binding. Firstly, the internalization rates of two types of TCRm antibodies and their conjugates were detected by flow cytometry. As shown in Figure 2A, about 50%–65% of the TCRm antibodies and conjugates were internalized by K562-A2-WT1 126-134 after a 2-hour incubation. The internalizations of ESK-MMAE and Q2L-MMAE were slightly reduced after conjugation, which is consistent with the results of affinity evaluation.
Moreover, the internalization of TCRm antibodies and their conjugates was verified via fluorescent confocal microscopy. TCRm antibodies or TCRm-ADCs are co-incubated with K562-A2-WT1 126-134, and labeled with Cy5-tagged human Fc antibodies and Cy3-tagged lysosomal associated membrane protein-1 (LAMP-1, a lysosomal marker). As shown in Figure 2B, both TCRm antibodies and TCRm-ADCs were observed in cells, indicating they can all be internalized by K562-A2-WT1 126-134. There is partial overlap of Cy5 and Cy3 fluorescence, suggesting that after internalization into the cells, TCRm antibodies and TCRm-ADCs can be transported to the lysosomes for degradation, thus releasing MMAE molecules.
Figure 2. Cellular internalization of TCRm antibodies and their conjugates (Shen Y, 2019)
View all MMAE-linker complex products
| Cat.No. | Product Name | Price |
| ADC-S-005 | Mc-MMAE | Inquiry |
| ADC-S-007 | Vc-MMAE | Inquiry |
| ADC-S-008 | OSu-Glu-vc-PAB-MMAE | Inquiry |
| ADC-S-017 | DBCO-PEG4-vc-PAB-MMAE | Inquiry |
| ADC-S-025 | MC-betaglucuronide-MMAE-1 | Inquiry |
| ADC-S-026 | MC-betaglucuronide-MMAE-2 | Inquiry |
| ADC-S-033 | Gly3-vc-PAB-MMAE | Inquiry |
| ADC-S-060 | MAL-di-EG-Val-Cit-PAB-MMAE | Inquiry |
| ADC-S-073 | mDPR-Val-Cit-PAB-MMAE | Inquiry |
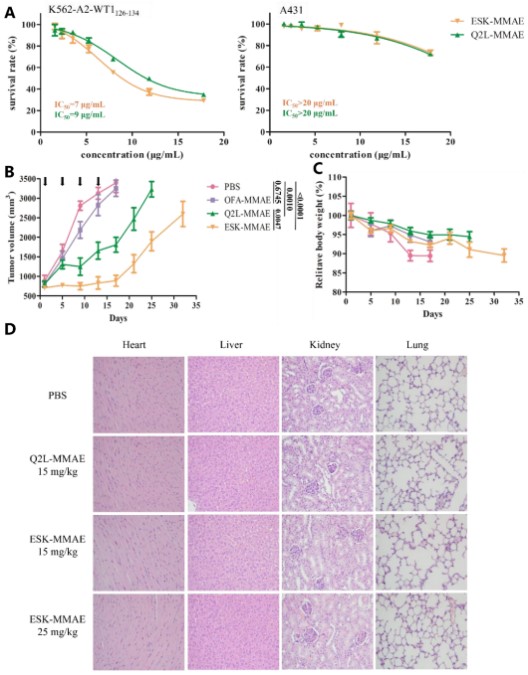
In vitro anti-tumor activity of TCRm-ADC
WT1 RMF/HLA-A*02:01 complex positive and negative cell lines were co-incubated with ESK-MMAE and Q2L-MMAE for 96 hours. Interestingly, compared with negative cells (A431), ESK-MMAE and Q2L-MMAE showed specific killing effects on K562-A2-WT1 126-134 (Figure 3). Although the affinity of ESK-MMAE is higher than that of Q2L-MMAE, both TCRm-ADCs show similar anti-tumor activity against K562-A2-WT1 126–134, and the IC 50 of ESK-MMAE and Q2L-MMAE are 7 and 9 μg/mL, respectively, which may be caused by the quantity of pHLA on the surface of K562-A2-WT1 126-134 cells.
Figure 3. Antitumor activity and toxicity of TCRm-ADCs (Shen Y, 2019)
In vivo efficacy evaluation of TCRm-ADC
Firstly, a xenograft model of K562-A2-WT1 126-134 leukemia was established in BALB/c nude mice, followed by the administration of TCRm or TCRm-ADC, with anti-CD20-ADC (Ofatumumab (OFA)-MMAE) used as a negative control. As shown in Figure 3B, the average tumor volume of the PBS group and the OFA-MMAE group (15 mg/kg) quickly reached 3000 mm3, and there was no significant difference in the average tumor volume between the two groups. In contrast, Q2L-MMAE and ESK-MMAE (15 mg/kg) significantly delayed tumor growth, suggesting that Q2L-MMAE and ESK-MMAE exhibited specific antitumor activities in vivo. Also, the phenomenon of rapid tumor growth after the termination of TCRm-ADCs treatment indicated that the two TCRm-ADCs possess tumor suppressive functions. Moreover, the antitumor activity of ESK-MMAE was significantly superior to Q2L-MMAE.
Meanwhile, changes in body weight were monitored to assess the in vivo toxicity of TCRm-ADC. Although a slight decline was noted in the relative body weight of all groups, the change in weight for mice treated with TCRm-ADC was smaller than that for mice treated with PBS, suggesting that mouse weight was largely influenced by the tumor burden. For a systemic toxicity evaluation, mice were euthanized 10 days after drug administration and the histological sections of major organs (heart, liver, kidney, and lung) were examined after staining with hematoxylin and eosin. No obvious histopathological changes were observed in the organ sections (Figure 4.). These results suggest that ESK-MMAE and Q2L-MMAE exhibit antitumor activity without any visible toxicity.
Preparation and Characterization of Bi-TCRm-ADC
To enhance the efficacy of TCRm-ADC, the “knob-to-holes” approach (which improves the success rate of non-homotypic antibody chain assembly) was used to generate double specific complex IgG against the WT1 RMF/HLA-A02:01 complex and NY-ESO-1 SLL/HLA-A 02:01 (Figure 5A). Following co-transfection of 293F cells with “knob” and “hole” plasmids, the bi-TCRm antibody was purified through Protein A antibody affinity chromatography and Ni-NTA affinity chromatography, which yielded a purified stable heterodimer.
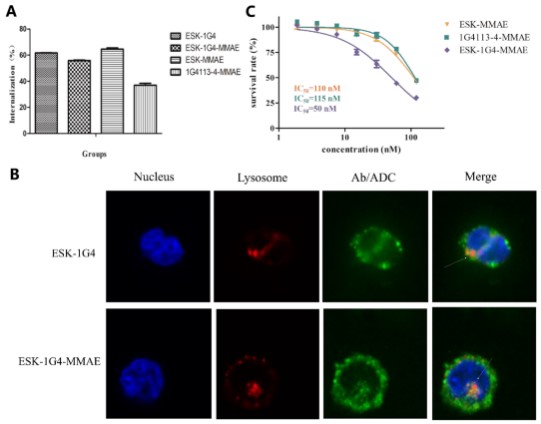
Internalization and in vitro cytotoxicity of Bi-TCRm-ADC
The internalization efficiency of ESK-1G4-MMAE was slightly lower than that of ESK-1G4 and ESK-MMAE, but significantly higher than that of 1G4113-4-MMAE (Figure 4A). In addition, the subcellular transport and localization of ESK-1G4-MMAE in K562-A2-NY-ESO-1 157-165 were further determined by fluorescence confocal microscopy (Figure 4B). After incubating with cells for 4 hours, ESK-1G4-MMAE was located inside the cytoplasm, indicating internalization. Furthermore, the internalized ESK-1G4-MMAE co-localized with LAMP-1, suggesting that ESK-1G4-MMAE can be transported to lysosomes to release MMAE molecules after internalization.
In the in-vitro antitumor activity study, ESK-1G4-MMAE demonstrated stronger efficacy against K562-A2-NY-ESO-1 157-165 compared to ESK-MMAE and 1G4113-4-MMAE (Figure 4C). This suggests that the antitumor activity of TCRm-ADC can be effectively improved by constructing bispecific antibodies to increase epitope binding density.
Figure 4. Internalization and in vitro cytotoxicity of ESK-1G4-MMAE (Shen Y, 2019)
WT1 Protein Epitope Peptide Prediction
In order to find the potential presentation peptides of WT1 protein, we used the online prediction algorithm to predict the epitope peptides of WT1 protein. According to the NetMHC binding prediction algorithm, there are 12 WT1 protein candidate peptides with a length of 8–11 and a suitable affinity with HLA-A*02:01. Subsequently, these 12 peptides were validated in terms of the human proteasome cutting site and TAP transport efficiency in NetCTL. Apart from the RMF epitope peptide, other WT1 protein peptides could potentially be presented by HLA I class molecules as targets for cancer treatment, reflecting the advantage of the epitope diversity of Bi-TCRm-ADC.
The enhanced potency of Bi-TCRm-ADC represents a promising strategy to enhance the anti-tumor action of TCRm-ADC. These new generations TCRm-ADC and Bi-TCRm-ADC will expand the use of intracellular cancer proteins and ADCs.
Reference
Shen Y, Li Y M, Zhou J J, et al. The antitumor activity of TCR-mimic antibody-drug conjugates (TCRm-ADCs) targeting the intracellular Wilms tumor 1 (WT1) oncoprotein[J]. International Journal of Molecular Sciences, 2019, 20(16): 3912.