In a recent study, researchers from the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ), the Weizmann Institute of Science in Israel, the University of South Florida, and the University of Texas MD Anderson Cancer Center found that the gut microbiome may modulate the efficacy of CAR-T cell therapy in treating patients with B-cell lymphoma. Personalized gut microbiome information obtained from patients’ gut microbiome prior to initiating CAR-T cell therapy treatment enables an accurate prediction of the subsequent responsiveness to such therapy, provided these patients are not pre-treated with broad-spectrum antibiotics. The findings were published online on March 13, 2023, in Nature Medicine with the title “A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy”.
Growing evidence from human studies and preclinical trials suggests that the gut microbiome may modulate the efficacy of T cell-driven cancer immunotherapies, such as immune checkpoint blockade. CD19-targeted CAR-T cell therapy offers a new treatment option for patients with certain forms of refractory and relapsed B-cell leukemia or lymphoma. But this immunotherapy is hampered by considerable heterogeneity in terms of responsiveness. At most, only 40% of patients achieve complete and long-term remission.
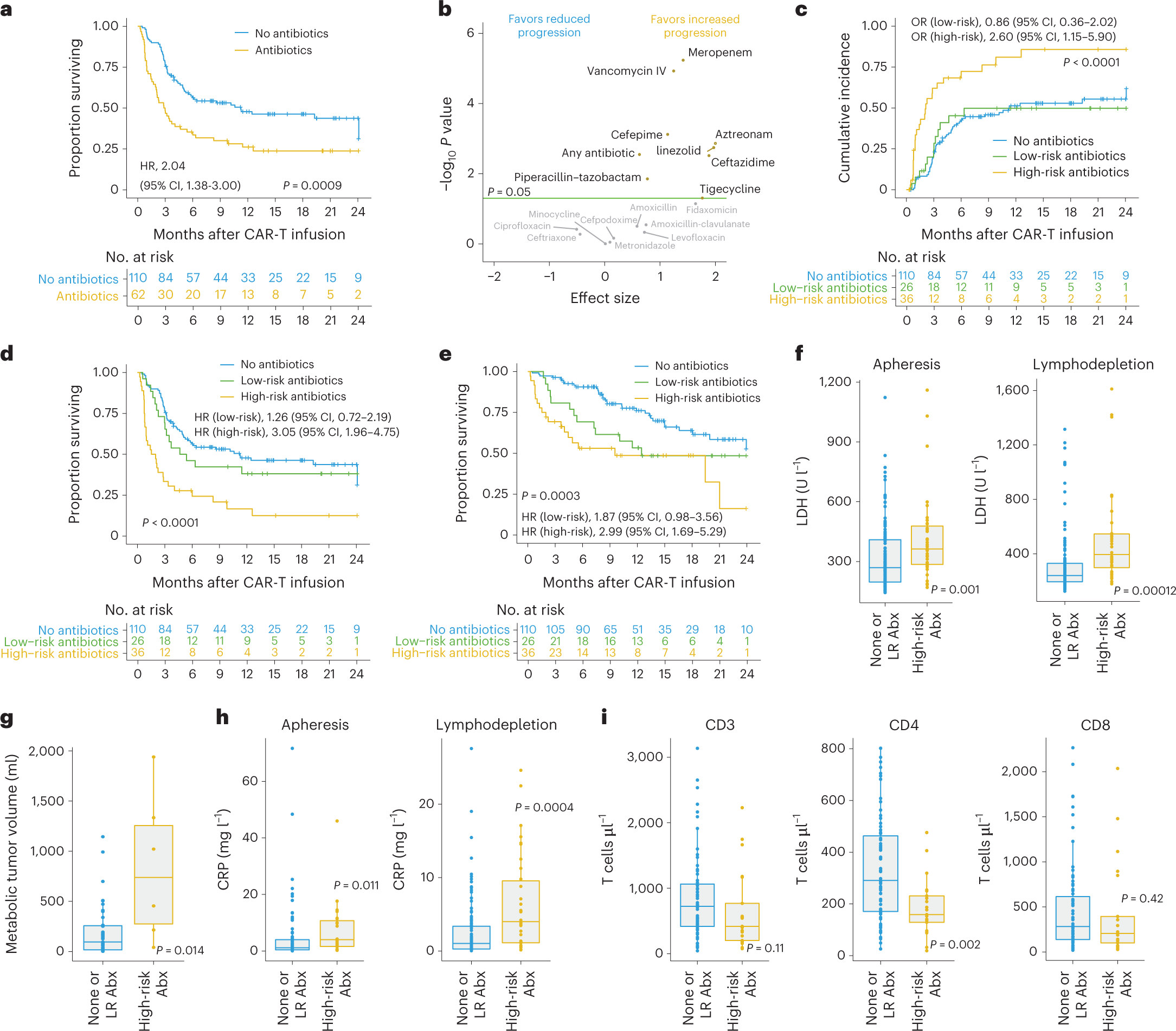
In this study, the authors found that the gut microbiome may modulate the efficacy of CD19-targeted CAR-T cell therapy in the treatment of patients with B-cell leukemia and lymphoma. The largest prospective study followed 172 lymphoma patients who had failed multiple rounds of prior chemotherapy from before the initiation of CAR-T cell therapy until two years after initiation. Interestingly, 20% of patients treated with broad-spectrum (“high-risk”) antibiotics such as meropenem, piperacillin-tazobactam, or cefepime had an altered clinical response to subsequent CAR-T cell therapy compared with patients who have been treated with other antibiotics and who were not treated with any antibiotics prior to treatment.
However, this reduction in antibiotic-related response to CAR-T cell therapy was not driven by the effects of antibiotics per se, but rather by the fact that patients who received “high-risk” antibiotics prior to initiating CAR-T cell therapy tended to have a higher pretreatment tumor load and systemic inflammation. These unfavorable pre-treatment conditions make subsequent CAR-T cell therapy less effective.
Importantly, the exclusion of these confounding “high-risk” antibiotic-treated patients from the analysis allowed the authors to identify a strong and previously obscured association between the gut microbiome of patients prior to CAR-T cell therapy and subsequent clinical response to immunotherapy, including patient survival.
To further strengthen the link between the initial gut microbiome prior to treatment and CAR-T cell efficacy independent of geographic, dietary, and other “local” confounding factors, the authors next used machine learning models trained on German patients and then validated on US patients. Importantly, these models were effective in predicting treatment outcomes, but only after excluding patients treated with “high-risk” antibiotics.
In other words, this study suggests that the pretreatment gut microbiome of lymphoma patients can help predict their response to subsequent CD19-targeted CAR-T cell therapy unless their microbiome is disrupted by broad-spectrum antibiotics. The authors identified several key gut microbiome features that can predict CAR-T cell efficacy, including Bacteroides, Ruminococcus, Eubacterium, and Akkermansia species. Among them, Akkermansia was also found to be associated with higher baseline peripheral T-cell levels in these patients.
Overall, this new study reveals a strong link between the gut microbiome and CAR-T cell therapy outcomes. Christoph Stein-Thoeringer, the co-first author of the paper, believes this may contribute to the development of a gut microbiome-based prediction method for CAR-T cell therapy outcomes.
In addition, these findings may lead to a better understanding of the different CAR-T cell activation, persistence, and clinical efficacy in different patients. The study also highlights the need to further explore the causal relationship between the gut microbiome and cancer immunotherapy outcomes.
Dr. Eran Elinav, the co-corresponding author of the paper, said, “These exciting findings exemplify the potential of our unique gut microbiome profile to be exploited as a possible marker of response to multiple human diseases and therapies, including cancer. With further research, we hope that gut microbiome-based diagnostic and therapeutic approaches will be incorporated into the field of precision oncology.”
Reference
1. Stein-Thoeringer, Christoph K., et al. “A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy.” Nature medicine 29.4 (2023): 906-916.