Since the world’s first chimeric antigen receptor (CAR)-T cell therapy was approved for marketing in 2017, the Food and Drug Administration (FDA) has approved a total of six CAR-T cell therapies to revolutionize the treatment of blood-based cancers. However, for more common solid tumors such as lung and breast cancer, CAR-T remains ineffective.
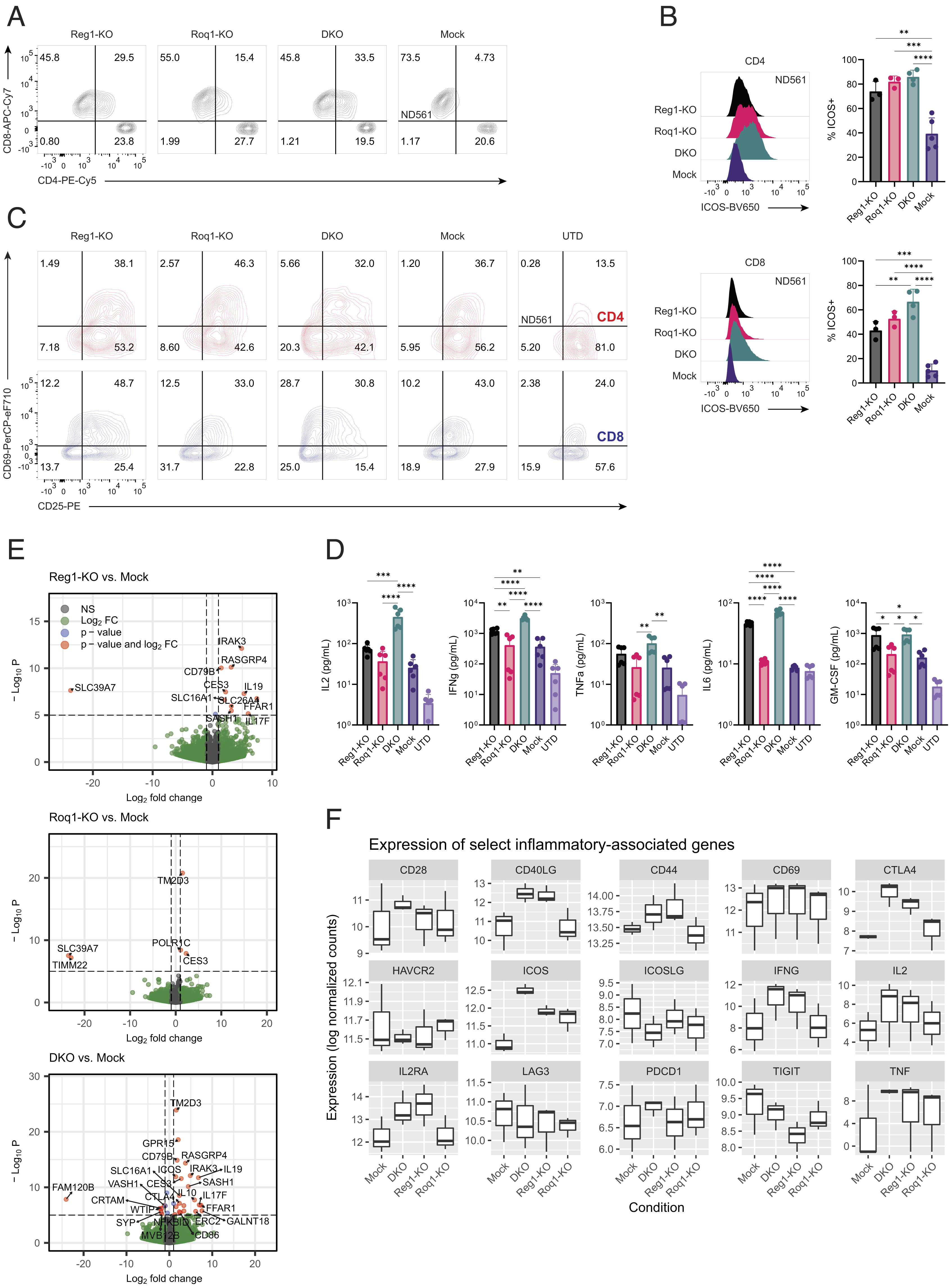
On March 15, 2023, Carl June’s team at the University of Pennsylvania published a paper in the Proceedings of the National Academy of Sciences (PNAS) titled “Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells”. The study developed a new combinatorial approach to help T cells attack solid tumors by knocking down two T cell inflammatory regulators, Regnase-1 and Roquin-1, via CRISPR-Cas9. This increases T cell expansion levels by at least 10-fold, thereby enhancing antitumor immune activity and persistence. These results suggest that immune inflammatory regulators may be promising targets for enhancing the anti-tumor response of CAR-T or T cell receptor-engineered T cell (TCR-T).
Professor Carl June, the corresponding author of the paper, hopes to develop available CAR-T cell therapies for patients with solid tumors, including the most common cancer types. This new study suggests that targeting immune inflammatory modulators to enhance the potency of T cells warrants further investigation.
The major challenge facing CAR-T cell therapy in solid tumors is T cell exhaustion, where continuous antigen exposure of tumor cells from solid tumors depletes T cells to the point where they are unable to generate an anti-tumor response. Using depleted T cells from solid tumor patients for CAR-T cell therapy is associated with poorer outcomes because these T cells are unable to expand effectively and “remember” their task to kill tumor cells.
Previous observational studies have suggested that the inflammatory regulator Regnase-1 is a potential target to indirectly overcome the effects of T-cell depletion by causing excessive inflammation when T cells are destroyed, allowing them to recover to generate an anti-tumor response. The team speculated that inhibiting another related but independent regulator of inflammation, Roquin-1, may further promote an anti-tumor response.
The team studied two clinical-stage T-cell therapies, mesCAR-T, a CAR-T cell therapy targeting mesothelin, and NYESO TCR-T, a TCR-T therapy targeting human esophageal squamous epithelial carcinoma antigen 1. Using CRISPR-Cas9 gene editing technology to knock down their Regnase-1 or Roquin-1, respectively, or simultaneously. The CRISPR-Cas9-edited engineered T cells were expanded and entered into a solid tumor mouse model, and the team observed that knockdown of either Regnase-1 or Roquin-1 improved the efficacy of CAR-T or TCR-T. While simultaneous knockdown was able to expand engineered T cells at least 10-fold, it also increased anti-tumor immune activity and engineered T cell lifespan, delivering optimal anti-tumor effects, with Regnase-1 being the main contributor.
Restricted expansion of CAR-T cells is often seen in solid tumor therapy, and the ability to make these T cells more effective and help gate massive expansion in large numbers would allow T cell therapies to better attack solid tumors.
David Mai, Ph.D., the first author of the paper, introduced that each of these two inflammatory regulators is involved in limiting the T-cell inflammatory response, and inhibiting them both produces a more potent anti-cancer effect than inhibiting one alone. Based on previous research, this study brings researchers closer to a promising CAR-T cell therapy strategy in the solid tumor setting.
Reference
1. Mai, David, et al. “Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells.” Proceedings of the National Academy of Sciences 120.12 (2023): e2218632120.