High-Throughput Exosomal Lectin Chip Detection Service
Overview Services Features FAQs
Creative Biolabs offers a service to detect glycosylated exosomes with lectin microarrays, allowing for high-throughput resolution of specific glycan structures in exosomes.
Overview of Lectin Microarray Detection
-
Lectin microarray technology enables the creation of high-throughput arrays by immobilizing a variety of traditional lectins on 3D polymeric chip substrates. This approach has revolutionized glycan structure analysis, transitioning from "one-to-one" to "many-to-many" interactions, allowing for the simultaneous screening and cross-validation of multiple glycans. This innovative tool offers valuable insights for researchers investigating exosomal protein glycosylation.
-
The application of lectin microarrays to biomarker development protocols, which integrate protein microarrays and mass spectrometry, provides new research ideas for marker development in a variety of complex diseases. This started with exosomes and their cellular low expression of glycoconjugates, deciphering the mechanism of exosomes mediating cancer cell metastasis and identifying relevant glycosylated exosome markers.
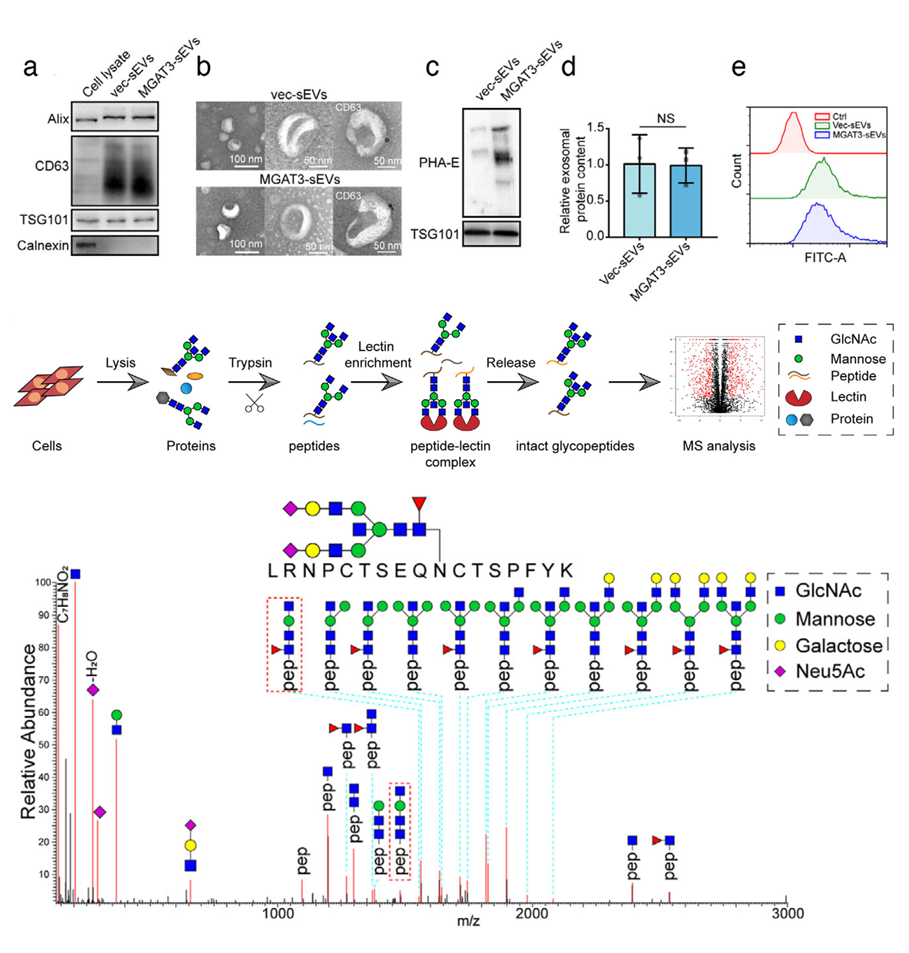 Fig. 1 Identification of integrin β1 as the target protein bearing bisecting GlcNAc.1
Fig. 1 Identification of integrin β1 as the target protein bearing bisecting GlcNAc.1
High-Throughput Exosomal Lectin Chip Detection Service at Creative Biolabs
At Creative Biolabs, glycan structure profiling based on lectin microarray technology has been developed for multi-directional glycan profiling, including specific protein surface glycan profile construction and differential detection, live cell surface glycan profile construction and differential detection, cell/tissue lysate glycan profile construction and differential detection, clinical sample glycan profile construction and biomarker discovery, and coupling with mass spectrometry technology to study glycosylated proteome.
More complex research projects can be accomplished by the combination of high-throughput lectin microarray and exosomal glycosylated proteome mass spectrometry. The exosomal glycoproteins identified by specific lectins are then resolved with glycosylated proteomic mass spectra to obtain a complete structure of the exosomal glycoprotein profile and glycosylation site map.
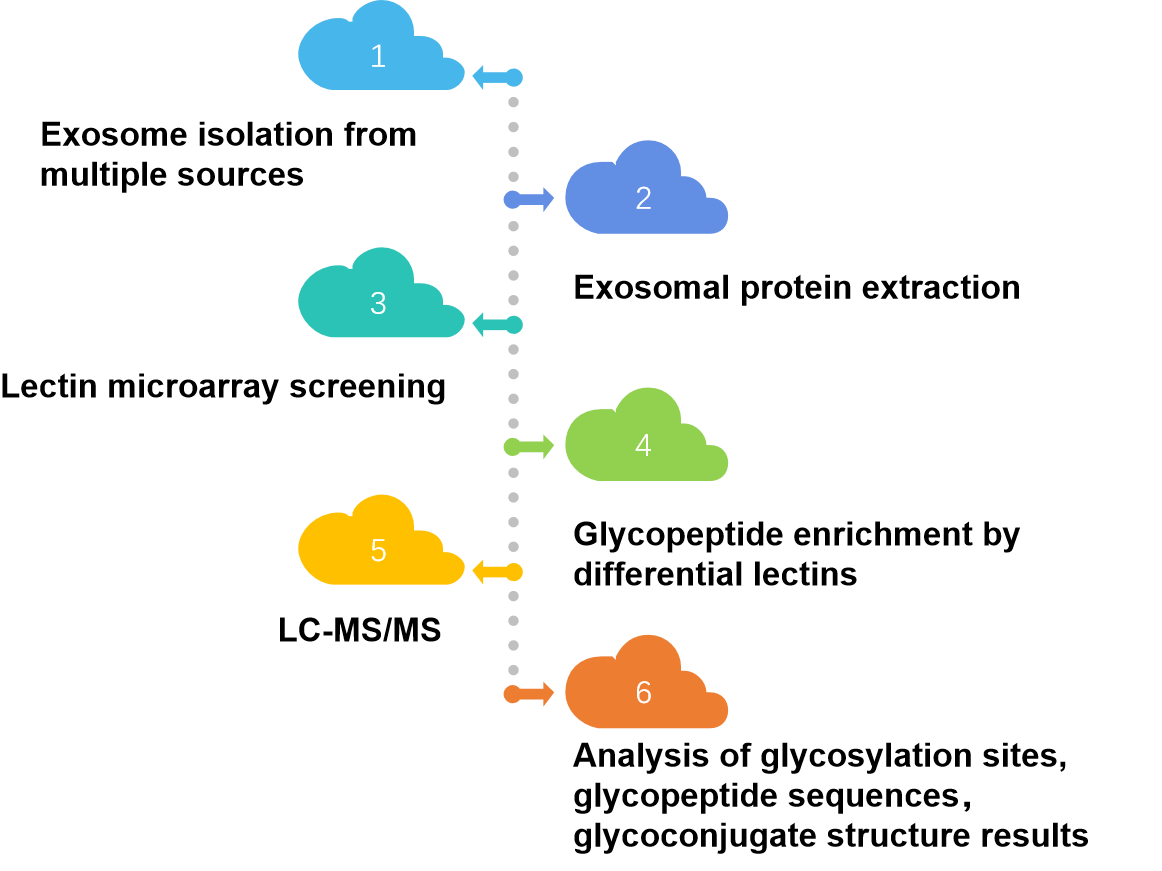 Fig. 2 High-throughput exosomal lectin chip detection workflow.
Fig. 2 High-throughput exosomal lectin chip detection workflow.
Features
-
Comprehensive Exosome Profiling: Our lectin chip technology enables the simultaneous detection and profiling of a broad spectrum of exosomal glycans, providing in-depth insights into the glycosylation patterns of exosomes from various sources.
-
High-Throughput Capability: Designed for large-scale studies, our platform supports the parallel analysis of multiple samples.
-
Sensitive and Specific Detection: Leveraging advanced lectin-glycan interactions, our service offers highly sensitive and specific detection of exosomal markers, ensuring accurate and reliable results even at low sample concentrations.
-
Customizable Assays: Tailored to meet the unique needs of your research, our service allows for the customization of lectin panels to target specific glycan structures relevant to your study.
-
Versatile Sample Compatibility: Our platform is compatible with a wide range of biological samples, including purified exosomes derived from multiple species, offering flexibility in sample selection.
FAQs
Q: What is a High-Throughput Exosomal Lectin Chip Detection Service?
A: Our High-Throughput Exosomal Lectin Chip Detection Service is a cutting-edge technology that allows for the rapid and accurate detection of exosomal protein glycosylation using a specialized microarray chip.
Q: How does the service work?
A: Our service involves isolating exosomes from biological samples, labeling them with lectin probes, and then analyzing them on our microarray chip. This makes it possible to identify several exosomal proteins at once in a high-throughput manner.
Q: How can I submit my samples for analysis?
A: To submit samples for analysis, please contact us to know about sample shipment and discuss your specific project requirements.
Creative Biolabs provides high-throughput exosome lectin microarray services to support the development of glycosylation profiles and their associated biomarkers. Please contact us to learn more.
Reference
-
Tan, Zengqi, et al. "Bisecting GlcNAc modification diminishes the pro‐metastatic functions of small extracellular vesicles from breast cancer cells." Journal of extracellular vesicles 10.1 (2020): e12005. Under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Identification of integrin β1 as the target protein bearing bisecting GlcNAc.1
Fig. 1 Identification of integrin β1 as the target protein bearing bisecting GlcNAc.1
 Fig. 2 High-throughput exosomal lectin chip detection workflow.
Fig. 2 High-throughput exosomal lectin chip detection workflow.









