Lentiviral Vector Development Service
The development of effective clinical protocols relies on safe and efficient gene delivery. Lentiviruses, a subgroup of the retrovirus family, have become pivotal tools for modern gene therapy due to their unique ability to transduce both proliferating and quiescent cells, ensuring broad and persistent therapeutic action. Building on this inherent advantage, we engineer these vectors to be the definitive vehicle for delivering sophisticated synthetic gene networks. These networks carry the genetic blueprints for a complete 'sense-and-respond' system, featuring highly specific receptors to detect pathological markers, an internal transducing cascade to process the signal, and powerful effector molecules to enact the final therapeutic response—be it shRNA, cytokines, or pro-apoptotic cues.
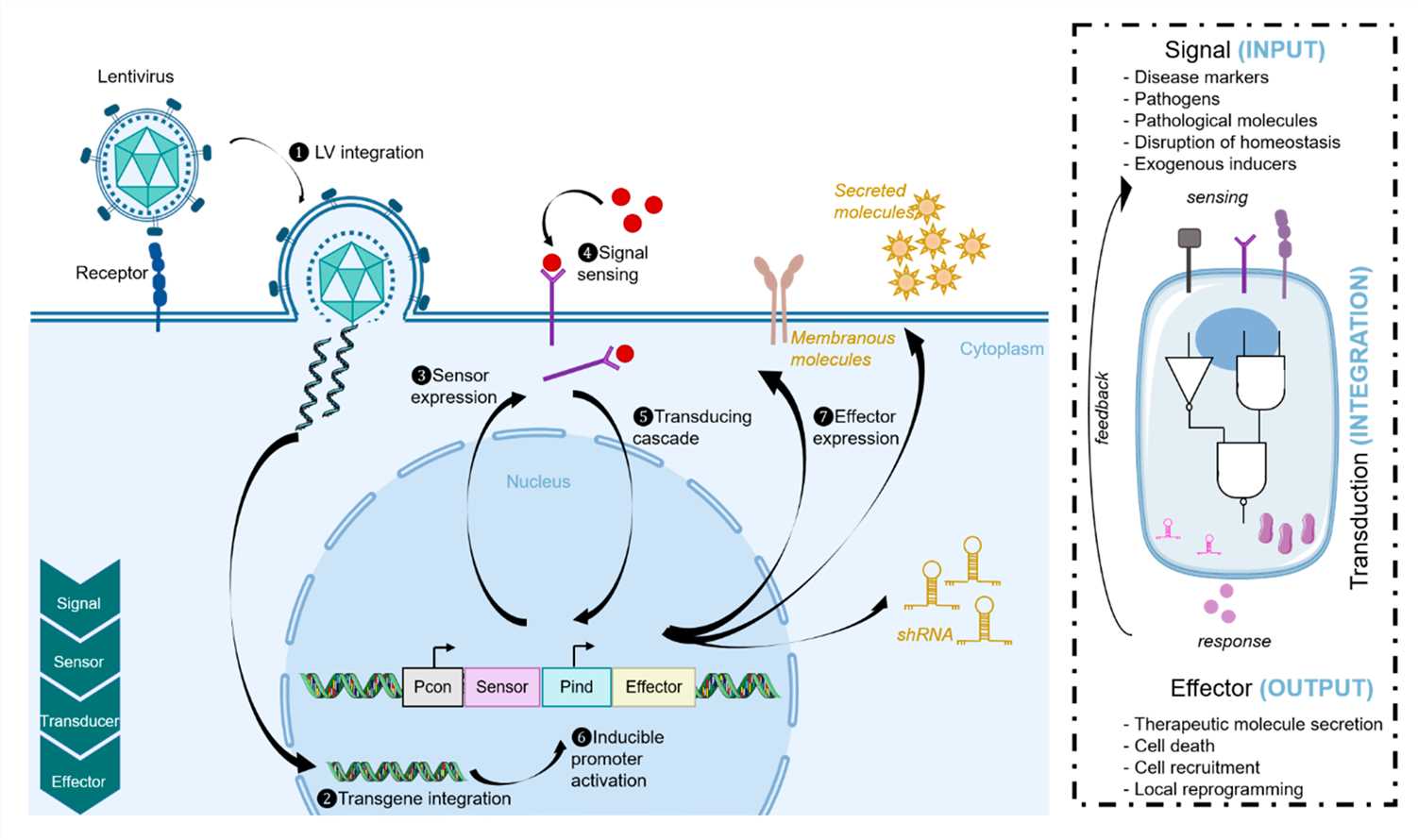 Fig.1 Lentiviral vectors are used to transduce cells1,2
Fig.1 Lentiviral vectors are used to transduce cells1,2
At Creative Biolabs, we provide comprehensive lentiviral vector solutions featuring multiple cutting-edge modifications designed for your advanced studies. From concept to clinic, our team is your dedicated partner, delivering the reliable, scalable, and high-quality vectors essential for accelerating your entire gene therapy pipeline.
Your Strategic Advantage in Gene Therapy
Navigating the complexities of vector production shouldn't slow your progress. We provide the expertise, technology, and genuine partnership to help you overcome challenges and achieve your milestones faster.
Collaborative Scientific Partnership
Our PhD-level experts act as an extension of your team, providing proactive consultation on vector design, optimization, and regulatory strategy.
Seamless Scalability
Our platform is built for growth. We ensure consistent vector quality and performance from small-scale research batches to large-scale pre-clinical production.
Uncompromising Quality & Safety
With a "safety-first" design philosophy and rigorous QC, we deliver vectors that meet the stringent requirements for pre-clinical and clinical development.
Timeline-Driven Project Management
We understand that speed is critical. Our dedicated project managers ensure transparent communication and on-time delivery to keep your program on track.
Workflow: A Clear Path from Concept to Delivery
Our transparent, phase-gated process ensures quality and consistency at every step, providing you with clear milestones and predictable outcomes.
Phase 1: Design & Construction
Collaborative design, gene synthesis, codon optimization, and production of high-purity plasmid DNA.
Phase 2: Production & Purification
Scalable production (Research or Pre-clinical grade) using optimized protocols and advanced purification strategies.
Phase 3: QC & Delivery
Rigorous, multi-assay QC testing, complete with a detailed Certificate of Analysis (CoA) and on-time delivery.
A Flexible Suite of Services
We offer a comprehensive menu of services designed to support your project at any stage, from initial vector design to large-scale production.
Vector Optimization Service
Fine-tuning vector components for enhanced titer, expression, and specificity.
Vector Design for Regulated Expression
Custom design of vectors with inducible or tissue-specific promoters.
Safety Determination Service
Comprehensive assays to assess vector safety, including RCL testing.
Vector Titration Service
Accurate quantification of viral vectors by functional and physical methods.
Vectors for Basic Research
High-quality vectors for gene function studies, including shRNA-encoded products.
Vectors for Gene Therapy
Pre-clinical grade vectors manufactured with stringent quality control for in vivo studies.
Advanced Vector Technology Platform
Our platform integrates cutting-edge technologies to deliver vectors with superior performance, safety, and specificity.
Safety-first vector design
Our lentiviral vectors are engineered for maximum safety. We utilize third-generation systems containing only three essential HIV-1 genes (gag, pol, rev), minimizing homology to wild-type virus. All non-essential viral sequences are removed, leaving only the necessary cis-acting elements (LTRs, RRE) for efficient packaging. Pseudotyping with VSV-G not only broadens the host cell range but also significantly increases vector titer and stability, making our vectors robust and reliable tools for your research.
Precision targeting technologies
Achieving precise transgene delivery is critical for efficacy and safety. We offer multiple optimization methods:
Expression Control: Utilize tissue-specific promoters to restrict gene expression exclusively to your target cells.
miRNA-Mediated Silencing: Incorporate miRNA target sites to actively silence transgene expression in off-target cells, such as antigen-presenting cells, to enhance safety and reduce immunogenicity.
Advanced Systems: We also develop vectors based on HIV-2 and FIV for specialized applications, including chimeric systems for unique research needs.
Quality Isn't an Option—It's Our Guarantee
We provide full transparency and robust data to give you absolute confidence in the vectors you receive.
Our Comprehensive Release Panel
Every batch is released only after passing a rigorous, multi-assay QC panel.
- Functional Titer (Transduction Units/mL)
- Physical Titer (p24 ELISA & qPCR)
- Purity Assessment (SDS-PAGE)
- Sterility & Mycoplasma Testing
- Endotoxin Assay (LAL)
- Replication Competent Lentivirus (RCL) Assay
Certificate of Analysis (CoA)
A detailed CoA, including all QC data and methods, is provided with every shipment for your records and regulatory filings.
Enabling Breakthroughs Across Diverse Applications
Our vectors have a proven track record of success in the most demanding research and pre-clinical applications.
Central Nervous System (CNS)
With strong neural stem cell tropism, our vectors are ideal for CNS gene transfer. We support research in Alzheimer's, Parkinson's, and spinal cord injury, enabling robust pre-clinical validation.
Cell Therapy
We provide high-titer vectors essential for the efficient and stable transduction of primary immune cells, forming the foundation of your cell therapy manufacturing process.
Broad Tissue Targeting
Our vectors efficiently transduce a variety of critical tissues, including retinal, hepatic, and muscle cells, supporting a wide range of therapeutic areas and research models.
Basic Research & Target Validation
Rapidly create stable cell lines for gene overexpression or knockdown (shRNA), accelerating your target identification and validation workflows with reliable tools.
What Our Clients Say
We are dedicated to building long-term partnerships and take pride in delivering exceptional results. See what leading research institutions and biotech companies have to say about our services.

Frequently Asked Questions
Have questions? Find quick answers here. For more specific inquiries, please don't hesitate to contact us.
Q: What is the difference between "Research Grade" and "Pre-clinical Grade" vectors?
A: Research Grade vectors are ideal for in vitro studies and target validation. They are purified to remove cell debris but may contain some impurities. Pre-clinical Grade vectors undergo more rigorous purification, typically using chromatography, to achieve high purity suitable for in vivo animal studies. They also come with a more comprehensive QC panel, including tests like endotoxin and replication-competent lentivirus (RCL) assays.
Q: How do I choose the right promoter for my target cells?
A: Promoter selection is critical for successful gene expression. For broad, potent, and long-term expression in most cell types, including primary and stem cells, we generally recommend the EF1α promoter. For extremely high, but potentially transient, expression in certain cancer cell lines, CMV may be a suitable choice. For applications requiring cell-type specificity, we can incorporate a tissue-specific promoter. Our scientific team is happy to consult with you to select the optimal promoter based on your specific cell type and experimental goals.
Q: What is included with my order besides the vector itself?
A: Every vector order includes the specified quantity of high-titer lentivirus, conveniently aliquoted for you. Critically, you will also receive a comprehensive Certificate of Analysis (CoA) detailing all quality control data for your specific batch, including functional titer, physical titer, sterility results, and any other assays requested. This document is essential for your records and potential regulatory filings.
Q: Can you package any size of gene insert?
A: The packaging capacity of lentiviral vectors is a key consideration. While the theoretical limit is around 10kb for the entire transcript (from the 5' LTR to the 3' LTR), vector titer tends to decrease significantly as the insert size increases. For optimal results, we recommend keeping the total size of your expression cassette (promoter + gene of interest + other elements) under 6-7kb. We can provide consultation on strategies for larger genes if needed.
Q: Can I provide my own plasmid for virus production?
A: Yes, you can provide your own lentiviral transfer plasmid. To ensure a successful production run, we will first need to review the plasmid map to confirm it contains all the necessary components for packaging. We also perform our own quality control on the plasmid DNA you provide, including sequencing key regions, to guarantee the integrity of the final vector. Please contact our team to coordinate the plasmid submission and review process.
References
- Page, Audrey, Floriane Fusil, and François-Loïc Cosset. "Toward tightly tuned gene expression following lentiviral vector transduction." Viruses 12.12 (2020): 1427. https://doi.org/10.3390/v12121427
- Distributed under Open Access license CC BY 4.0, without modification.
