Adenovirus Vector
Adenoviral vectors have emerged as powerful tools in gene therapy research, offering exceptional advantages including high transduction efficiency, large packaging capacity, and robust gene expression. These versatile vectors provide remarkable flexibility in genetic modification while maintaining impressive safety profiles and manufacturing scalability. As the field continues to advance, researchers and developers seek reliable solutions to harness these capabilities effectively. At Creative Biolabs, we offer comprehensive adenoviral vector services spanning from sophisticated vector design and construction to scalable production and purification, empowering our partners with cutting-edge technology and deep expertise to accelerate their research and development goals. Our integrated platform combines innovative approaches with rigorous quality standards to deliver customized solutions that meet the evolving demands of gene therapy research.
UNDERSTANDING ADENOVIRAL VECTORS
Adenoviral vectors represent one of the most versatile and well-characterized gene delivery systems in molecular biology. While the structural and biological characteristics of adenoviruses provide the foundation for their utility (as shown in Table 1), their development into different vector generations has further expanded their applications and enhanced their safety profiles (detailed in Figure 1 and Table 2).
Table 1. Fundamental characteristics of adenoviruses
| Characteristic | Description |
|---|---|
| Viral Structure |
|
| Serotypes |
|
| Genome Organization |
|
| Cell Entry |
|
| Expression Pattern |
|
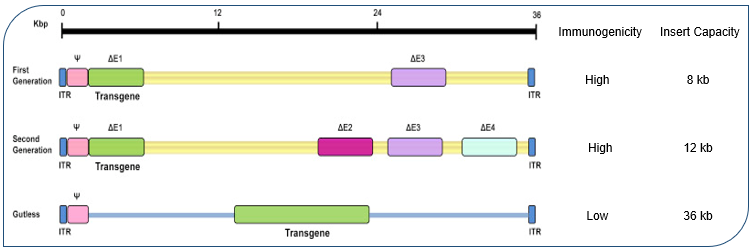 Fig.1 Adenovirus vector construction for gene therapy
Fig.1 Adenovirus vector construction for gene therapy
Table 2. Generations of adenoviral vectors: comparative analysis and applications
| Vector Generation | Deletion/Modification | Packaging Capacity | Key Features | >Main Applications |
|---|---|---|---|---|
| First Generation |
|
~8 kb |
|
|
| Second Generation |
|
~8 kb |
|
|
| Third Generation (HD-Ad) |
|
Up to 36 kb |
|
|
OUR EXPERTISE & SERVICES
We provide end-to-end adenovirus vector development solutions with a focus on flexibility and precision to match your exact research specifications. Following our standard workflow, our customizable platform adapts to your unique project requirements while maintaining exceptional quality standards, enabling you to achieve optimal results through personalized approaches backed by our extensive expertise.
Initial Design Phase
- Vector design strategy consultation
- Selection of optimal adenovirus serotype
- Transgene and promoter optimization
- Vector capacity assessment
- Regulatory element design
- Safety consideration review

Recombinant Adenovirus Construction
- Shuttle plasmid construction
- Insertion of transgene expression cassette
- Integration of regulatory elements
- Verification of genetic elements
- Quality control of intermediate constructs
Adenovirus Vector Production
- Transfection and viral rescue
- Initial viral stock generation
- Viral amplification
- Optimization of culture conditions
- Scale-up strategies

Adenovirus Vector Purification
- Crude lysate processing
- Chromatography purification
- Ultrafiltration/diafiltration
- Final formulation
- Concentration adjustment
Adenovirus Vector Characterization
- Viral titer analysis (physical and infectious titers)
- Comprehensive vector characterization (genome analysis, purity, stability)
- Expression verification and potency assessment
- Safety and purity testing (sterility, endotoxin, mycoplasma)
- Complete documentation with detailed analysis reports and CoA
Delivery & Support
- Optimized storage conditions and stability recommendations
- Professional handling and shipping guidance
- Comprehensive technical consultation and troubleshooting
- Expert support for data interpretation and usage
- Ongoing project assistance
Beyond our standard workflow, we offer specialized adenovirus vector development services to address diverse research needs and applications in vaccines and gene therapy. We also provide recombinant adenovirus vector products containing various promoters and shRNA-encoded genes. Explore our specialized services designed to meet your unique research requirements:
Adenovirus-Based cDNA Expression Libraries Generation Service
We construct comprehensive adenoviral cDNA expression libraries tailored to your research needs. Our service encompasses library design optimization, high-throughput cloning, quality-controlled vector production, and detailed characterization of the library complexity. We provide validated libraries with guaranteed coverage and diversity, supported by thorough documentation of library characteristics and screening protocols.
Helper-Dependent Adenoviral Vectors Service
We specialize in developing advanced helper-dependent adenoviral vectors with superior capacity and safety profiles. Our service covers the complete development process from vector design and helper virus system optimization to large-scale production and purification. We ensure high-quality HD-Ad vectors with minimal helper virus contamination, validated expression, and comprehensive quality testing.
Adenoviral Vector-based Vaccine Development
Our vaccine development service utilizes cutting-edge adenoviral vector technology to create effective vaccine candidates. We offer comprehensive solutions including antigen optimization, vector design, immunogenicity evaluation, and scalable production. Our platform supports both traditional and novel vaccination approaches, with extensive testing for safety, stability, and immune response.
Development of Adenoviral Vector as Immune Stimulant
We develop specialized adenoviral vectors designed to enhance immune responses. Our service includes vector modification for optimal immune stimulation, incorporation of immunomodulatory genes, and validation of immune activation. We provide detailed characterization of immune responses and support for mechanism-of-action studies.
Adenoviral Vector-based Suicide Gene Therapy Development
We offer complete development solutions for suicide gene therapy using optimized adenoviral vectors. Our service encompasses vector design with specific promoter systems, suicide gene optimization, safety feature integration, and validation of therapeutic efficacy. We ensure precise control of gene expression and thoroughly evaluate cell-killing efficiency.
Adenoviral Vector Design for RNAi Delivery
We design and develop specialized adenoviral vectors for efficient RNAi delivery. Our service includes custom shRNA/miRNA design, vector optimization for targeted delivery, expression validation, and knockdown efficiency assessment. We provide comprehensive solutions for both single and multiple RNAi target delivery systems.
Upstream Bioprocess Development for Adenovirus Vector
We optimize upstream processes for efficient adenoviral vector production. Our service covers cell line development, media optimization, culture parameter fine-tuning, and scale-up strategy development. We focus on maximizing vector yield while maintaining consistent quality through process validation and detailed documentation.
Downstream Process Development for Adenovirus Vectors
We develop robust downstream processes for adenoviral vector purification. Our service includes purification strategy design, chromatography method development, filtration optimization, and final formulation development. We ensure high purity, potency, and stability through comprehensive process validation and quality testing.
PLATFORMS & CAPABILITIES

Our state-of-the-art facilities are equipped with comprehensive analytical and production platforms that ensure exceptional quality and reliability in adenoviral vector development and manufacturing. At the heart of our purification and analysis workflow are advanced ÄKTA Pure/Avant FPLC and HPLC systems, enabling precise vector purification and sophisticated analysis. The production capability is powered by industrial-scale bioreactor systems (up to 200L) and automated cell culture systems, supported by Wave bioreactors for flexible scale-up operations, enabling efficient and scalable manufacturing from research to pilot scale. For downstream processing, we utilize sophisticated tangential flow filtration (TFF) systems and large-scale chromatography platforms to ensure optimal purification outcomes while maintaining vector integrity. Our analytical strength is further enhanced by cutting-edge flow cytometry and digital PCR systems, enabling precise characterization and quantification of viral vectors. This exceptional combination of advanced equipment is complemented by comprehensive supporting facilities including stability chambers, multiple centrifugation systems, and dedicated quality control instruments. Our integrated platform ensures consistent delivery of high-quality adenoviral vectors that meet the most rigorous standards for research and development applications, while providing the flexibility to accommodate diverse project requirements and scale-up demands.
TRUSTED BY INDUSTRY LEADERS


FREQUENTLY ASKED QUESTIONS
What are the available scales for adenovirus production and their typical applications?
We offer multiple production scales to meet different research and development needs:
- Research Scale (1-5L): Suitable for proof-of-concept studies and preliminary research
- Medium Scale (10-50L): Ideal for preclinical studies and process development
- Large Scale (100-200L): Designed for pilot production and scale-up validation
Each scale includes comprehensive process optimization, in-process testing, and detailed documentation. Production can be adjusted based on your specific titer requirements and application needs. We also provide consultation on scale selection based on your downstream applications and quantity requirements.
What starting materials do I need to provide for adenoviral vector development?
For standard vector construction, you need to provide either the transgene sequence information or the ready-to-use transgene plasmid. For sequence information, please provide the complete CDS with detailed annotation. For plasmids, we require 2-5 µg high-quality DNA with concentration ≥100 ng/µL and comprehensive sequence data. If you have specific requirements for promoters, regulatory elements, or vector backbones, please specify in advance. We can also assist with construct design and optimization, including codon optimization and regulatory element selection. All provided materials should be accompanied by detailed documentation including sequence verification, source information, and any relevant safety data.
What quality control parameters do you test for, and what documentation will I receive?
Our comprehensive QC testing package includes:
- Identity Testing: Transgene sequencing, restriction mapping
- Purity Analysis: SDS-PAGE, SEC-HPLC, residual host cell DNA/protein
- Biological Testing: Sterility, endotoxin, mycoplasma
- Potency Testing: Physical titer (vp/mL), infectious titer (IFU/mL)
- Function Verification: Transgene expression, specific activity
Each batch is delivered with a detailed Certificate of Analysis covering all test results, specifications, and methods. We provide complete documentation including:
- Development history and production records
- Analytical methods and validation reports
- Stability data and storage recommendations
- Raw data from key analyses
Additional customized testing can be performed upon request to meet specific requirements.
What viral titers and purity levels can I expect for different applications?
Our typical production achieves the following specifications:
| Parameters | Research Grade | Development Grade |
|---|---|---|
| Physical Titer | 1×1012-13 vp/mL | >1×1013 vp/mL |
| Infectious Titer | 1×1010-11 IFU/mL | >1×1011 IFU/mL |
| Purity (SEC-HPLC) | >90% | >95% |
| Host Cell Protein | <100 ng/mg | <50 ng/mg |
| Host Cell DNA | <10 ng/dose | <5 ng/dose |
Specifications can be customized based on your specific requirements. We implement comprehensive optimization strategies to achieve desired quality targets while maintaining high yields. Each batch undergoes thorough testing to ensure consistent quality and performance.
RESOURCES
Use the resources in our library to help you understand your options and make critical decisions for your study.
RELATED SECTIONS
Online Inquiry
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.