Herpes Simplex Virus Vector Development Service
Creative Biolabs offers a leading Herpes Simplex Virus (HSV) Vector Development Service, harnessing HSV's unique biological advantages for advanced gene therapy and biomedical research. Our service spans comprehensive design, generation, and purification of high-quality HSV vectors, including replication-defective, replication-competent (oncolytic), and amplicon types. We employ cutting-edge genetic engineering and optimized production protocols, ensuring high titers and purity.
Herpes Simplex Virus Introduction
Herpes simplex virus (HSV) is a highly cytotoxic human pathogen with a large and complex genome that encodes at least 80 gene products, making it not an ideal gene delivery vector at first glance. However, not all natural infections of HSV lead to cell death; In neurons, the virus establishes a latent infection during which the viral genome persists indefinitely without any significant adverse effects on the host cell. This natural neurotropic nature makes HSV an attractive candidate for neuronal gene delivery.
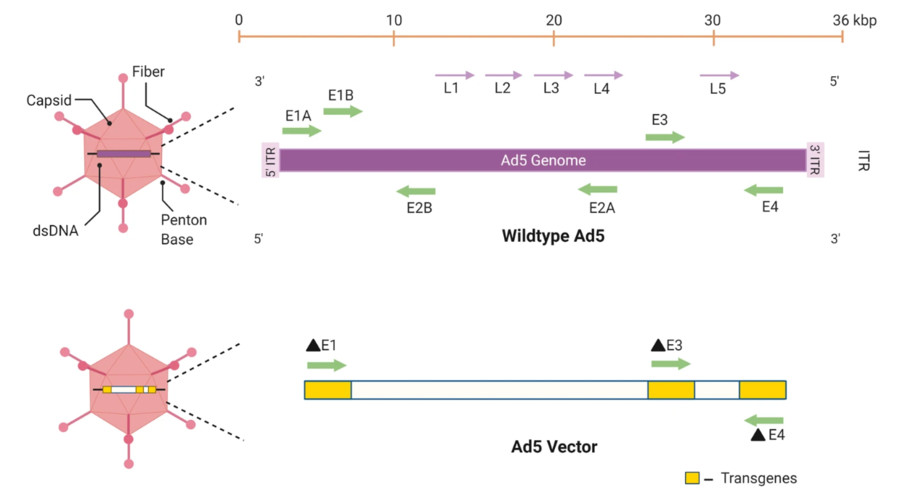 Figure 1 The adenovirus genome is characterized by inverted terminal repeats (ITR) and several early (E) and late (L) genes.1
Figure 1 The adenovirus genome is characterized by inverted terminal repeats (ITR) and several early (E) and late (L) genes.1
Herpes Simplex Virus Vector
HSV vector possesses a double stranded linear genome of 150kb and due to its neurotrophic nature, it has the greatest potential for gene delivery to the nervous system and tumor cells. The vector based on herpes simplex virus is mainly used for neuronal gene delivery, utilizing the natural neurotropic nature of the virus. There are two types of vectors to choose from: replication defective viruses, whose cytotoxicity has been eliminated by deleting viral gene products; Amplification vector, which is a plasmid packaged into HSV particles with the help of helper viruses.
Core Services at Creative Biolabs
Different applications have different requirements for vector systems. In most cases, the vector needs to completely inhibit replication and lacks all cytotoxicity, although some cancer "gene therapy" methods use mutated viruses to selectively replicate and kill malignant cells rather than non-malignant cells. Gene therapy to correct genetic defects requires long-term expression of genetically modified organisms and delivery of physiologically appropriate levels of protein to all affected cells. Cancer gene therapy typically only requires transient, high-level expression of a potential toxic product in malignant cells.
Gene Synthesis and Plasmid Construction
- Transgene Optimization
- Vector Backbone Selection
- Recombinant Plasmid Construction
Viral Vector Generation and Propagation
- Transfection and Helper System Utilization
- Virus Amplification
Purification and
Concentration
- Harvesting and Lysis
- Concentration
Key Advantages
Creative Biolabs offers comprehensive HSV vector services, from design and construction to purification, production, and quality verification, providing high titer and purity vectors for research and clinical use.
- Large Insertion Capacity
- Broad Host Range
- Long-Term Gene Expression
- Neurotropic Nature
- Low Toxicity
- Minimal Risk of Insertional Mutagenesis
- Advanced Vector Design
HSV Vector Applications
Cancer Immunotherapy
By expressing immune regulatory cytokines such as granulocyte/macrophage colony-stimulating factor (GM-CSF) within malignant cells, the host's immune response to tumors can be stimulated. Many different viral vectors are being developed as cancer vaccines, but a particularly attractive candidate vaccine is based on the gH-deficient DISC system mentioned above, as it has entered phase I/II clinical trials as a genital herpes vaccine.
Neuroprotection in Stroke
In order to utilize the natural neurotropic nature of viruses, HSV vectors are mainly designed to deliver genes to the nervous system. Among the many potential applications of gene therapy in the brain, perhaps the most attractive are those that require short-term, unregulated expression of genetically modified organisms at specific anatomical locations. These methods allow for a single injection using simple expression cassettes and vectors.
Parkinson's Disease
One approach is to use neuroprotective strategies to prevent the loss of dopaminergic neurons in the substantia nigra (SN). The replication defective HSV vector has been used to transiently express Bcl-2 and glial cell derived neurotrophic factor in rat SN with 6-hydroxydopamine injury. This resulted in a 50% increase in the number of surviving dopaminergic neurons in SN and a decrease in abnormal rotational behavior.
Pain
Peripheral inoculation with replication defective HSV vectors containing the proenkephalin gene controlled by the HCMV IE promoter resulted in the expression of proenkephalin in DRG neurons. In a rat model of inflammatory pain, the vector exhibited analgesic effects for up to 4 weeks and was reestablished after re inoculation. The analgesic effect has also been confirmed in models of arthritis, cancer bone pain, and neuropathic pain.
Frequently Asked Questions
Q1: What are the main advantages of using HSV vectors compared to other viral vectors such as AAV or lentivirus?
A1: The RSV carrier has several significant advantages. Compared to AAV (about 4.7kb) or lentivirus (about 8kb), they have greater cloning ability (up to 150kb) and can deliver multiple or very large therapeutic genes. Their natural neurotropic nature makes them an ideal choice for neuronal gene delivery, and they establish a non-integrated free latency, minimizing the risk of insertion mutations seen in integrated vectors such as lentiviruses. In addition, HSV vectors with replication ability have potent oncolytic properties against various cancers.
Q2: How do you ensure the safety of replicating defective HSV vectors in vivo?
A2: Safety is the most important. For HSV vectors with replication defects, we ensure safety by deleting essential viral genes (such as ICP4) that are crucial for virus replication in non-complementary cells. We utilize advanced non assisted systems, such as BAC based non assisted virus packaging, to prevent the generation of auxiliary virus contaminants with replication capabilities. Strict quality control includes sensitive detection to detect any potential replication capable revertant mutations, ensuring that the vector is safe for preclinical and potential clinical use.
Q3: Can your HSV vector achieve long-term gene expression?
A3: Yes, for applications that require long-term expression, especially in neurons, our replication defective and amplicon HSV vector aims to establish latency. During the incubation period, the viral genome exists as a free organism in the host cell nucleus, allowing for sustained expression of transgenes over a longer period of time, typically throughout the entire lifecycle of infected cells, without significant immune clearance or toxicity.
Q4: Can your HSV vector service be customized according to specific research needs?
A4: Of course. Our HSV vector development service is highly customizable. We work closely with our clients to understand their specific research objectives and provide tailored solutions for transgenic selection, promoter design, vector skeleton modification, purification strategies, and even specific quality control parameters. Our goal is to provide a vector that accurately meets your experimental requirements.
Contact Us Now
By virtue of the HSV vector technology platform, Creative Biolabs provides the target gene packaging design and construction services of replication-defective HSV vectors for its worldwide clients. Please feel free to contact us for more details and our scientists will conduct further in-depth discussion on your project.
Reference
- Travieso T, Li J, Mahesh S, et al. The use of viral vectors in vaccine development. npj Vaccines, 2022, 7(1): 75. https://doi.org/10.1038/s41541-022-00503-y (Distributed under Open Access license CC BY 4.0, without modification.)
