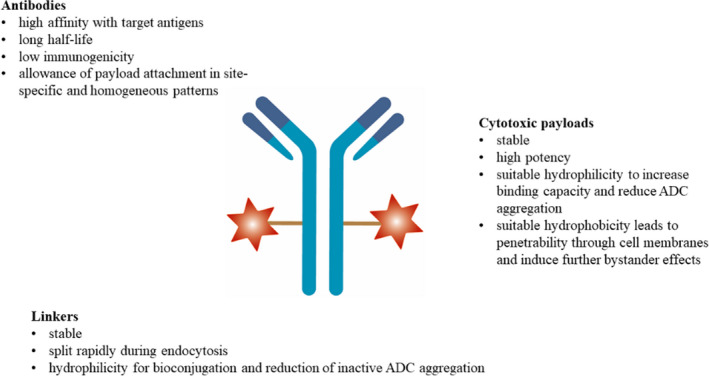
Basic Structure of ADCs
Antibody-drug conjugates (ADCs) are conjugates consisting of three basic components: monoclonal antibodies (which target specific tumor antigens), highly potent cytotoxic small molecule payloads, and linkers.
The basic structure of the ADC is shown in the figure below:
Fig.1 Basic characteristics of ADC. (Li W.Q., et al., 2021)
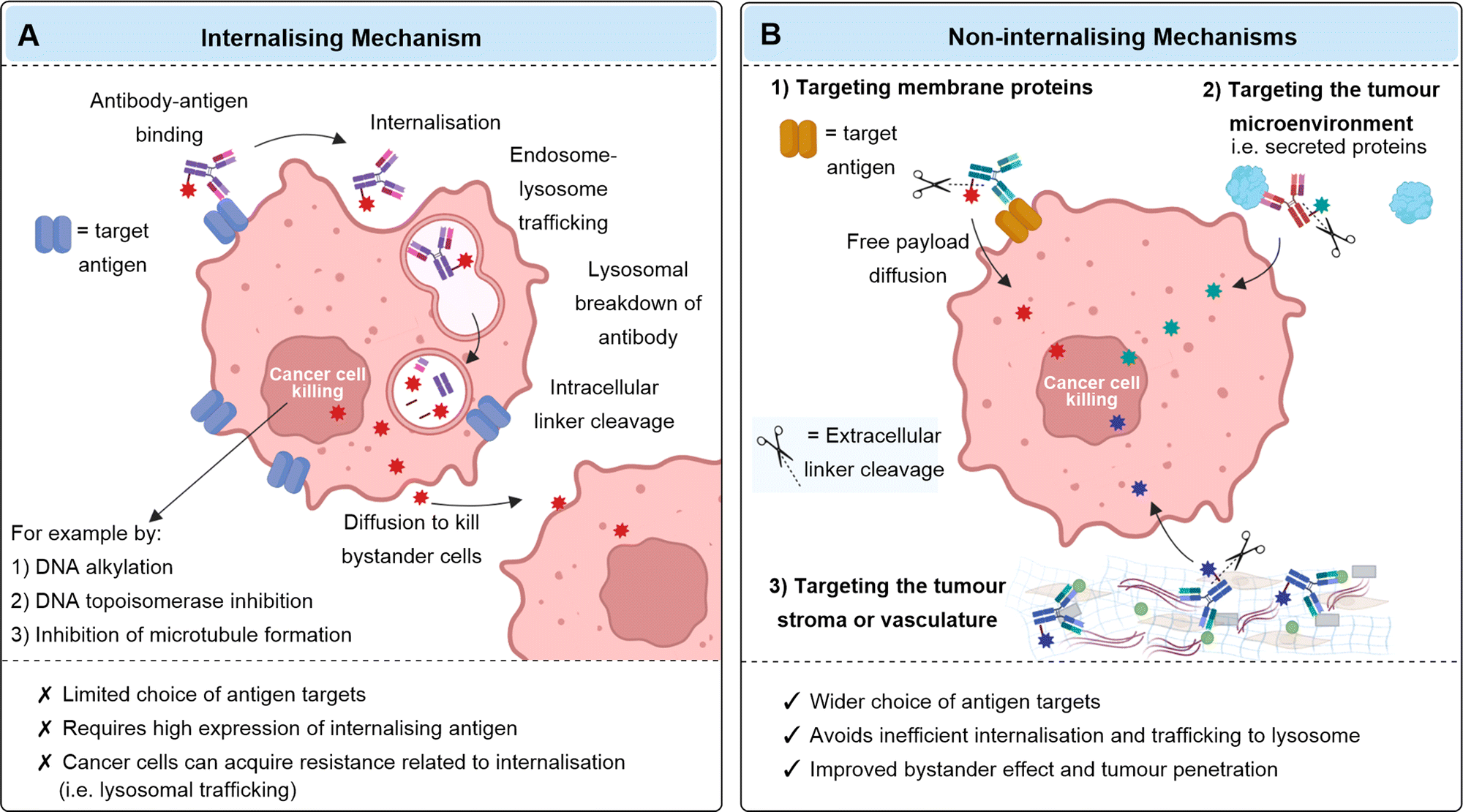
Mechanism of Action of ADC
The traditional mechanism of action of ADCs usually involves internalization. After intravenous injection, the antibody binds to the target antigen on cancer cells, and the ADC-antigen complex is endocytosed through antigen-dependent or antigen-independent pinocytosis. The ADC is degraded in lysosomes, releasing the cytotoxic payload. If the payload has appropriate membrane permeability, it can diffuse into surrounding “bystander” cancer cells (which may or may not express the target antigen) and exert its cytotoxic effects by damaging DNA or inhibiting microtubule assembly. Currently, FDA-approved ADCs are mostly internalizing antibodies.
Fig.2 (A) The mechanism of an internalizing ADC (B) the mechanism of non-internalizing ADCs. (Ashman, N., et al., 2022)
ADCs ultimately circulate in the bloodstream in three dynamic forms: conjugated form (the main portion), the naked antibody, and free payload molecules. The ratio of these three components can vary depending on incomplete coupling or linker instability during production.
Pharmacokinetics of ADCs
Unlike other small or large molecules, which usually only measure a few metabolites for pharmacokinetic analysis, ADCs require several components to characterize their PK profile. Understanding the pharmacokinetics of ADCs is critical to selecting safe and effective doses in patient populations.
Absorption
After administration, the drug enters the systemic circulation. Drugs are generally administered orally, intravenously, intramuscularly, and subcutaneously each with its own ADME characteristics as well as advantages and disadvantages. Most antibodies are usually given by intravenous bolus or infusion, but can also be administered subcutaneously. However, for ADCs, the current route of administration is intravenous injection or infusion. Subcutaneous administration of ADCs may not be feasible due to the reaction of the cytotoxic payloads and local deposition of cytotoxic agents.
Distribution
After drug administration, the drug is distributed throughout the body after absorption. Drug distribution is based on the biochemical properties of the drug. Distribution is a process of convection and diffusion, regulated by polarity, size, protein concentration, and the ability of the drug to bind to tissue. The distribution of a drug in the body can be described by its volume of distribution. Due to their size and polarity, the distribution of antibodies and ADCs is usually limited to blood vessels and intercellular space. The initial distribution of the ADC is usually confined to the blood compartment, and the volume of distribution is usually equal to the blood volume. Subsequently, ADCs can be distributed to the interstitial space. ADC distribution may also be affected by target antigen expression and internalization.
Unconjugated antibodies can distribute to non-target tissues through antigen-specific or non-specific mechanisms and may have little or no pharmacological effect. On the other hand, the distribution and accumulation of ADCs may lead to undesired (toxic) pharmacological effects due to the uptake of the ADCs and the subsequent release of cytotoxic drugs or their metabolites.
Metabolism and Clearance
The clearance of ADCs is very similar to that of monoclonal antibodies. ADC clearance is an accumulation process of deconjugation (loss of drug from ADC) and ADC degradation catabolism. ADCs are non-specifically degraded by proteolysis in many tissues such as liver, kidney, skin and muscle. Furthermore, pinocytosis results in antibody degradation by lysosomal enzymes. ADCs can also be cleared by target-mediated mechanisms, which can lead to saturable clearance pathways and non-linear PK of antibodies and ADCs.
Toxicity
The antibodies, linkers, and payloads in the ADCs structure all contribute to the toxicity of the ADC. In addition to cytoreductive and gastrointestinal adverse effects caused by the chemotherapeutic payload, each ADC drug has specific toxicities that are uncommon in oncology. Each ADC has subtle differences in ocular, pulmonary, dermatologic, and neurologic toxicities.
Structural modifications are sometimes performed to improve the PK properties of therapeutic proteins. The most common methods of modifying these agents are glycosylation and PEG modification. These modifications can reduce clearance and increase the half-life of the therapeutic proteins. Glycosylation enhance protein stability, regulate ligand-receptor interactions and protein secretion, and improve protein PK properties. PEGylation of ADC may help improve their solubility and reduce their aggregation.
Finally, traditional internalization mechanisms for ADCs have many limitations, such as dependence on the overexpression of internalized antigens. In addition, antibodies are large proteins that diffuse slowly and have limited tumor penetration. Non-internalizing mechanisms may expand the range of cancer targets, as they exclude the strict requirements for internalizing ADCs. In fact, some non-internalizing ADCs target most aggressive cancers and may be a more promising broad-spectrum anticancer approach.