Currently, the more common technical routes for lead antibody production mainly include hybridoma, antibody library, and single B cells. Among them, hybridoma technology and antibody library technology were applied earlier, and single B cell technology started slightly later and is relatively new.
Hybridoma Technology
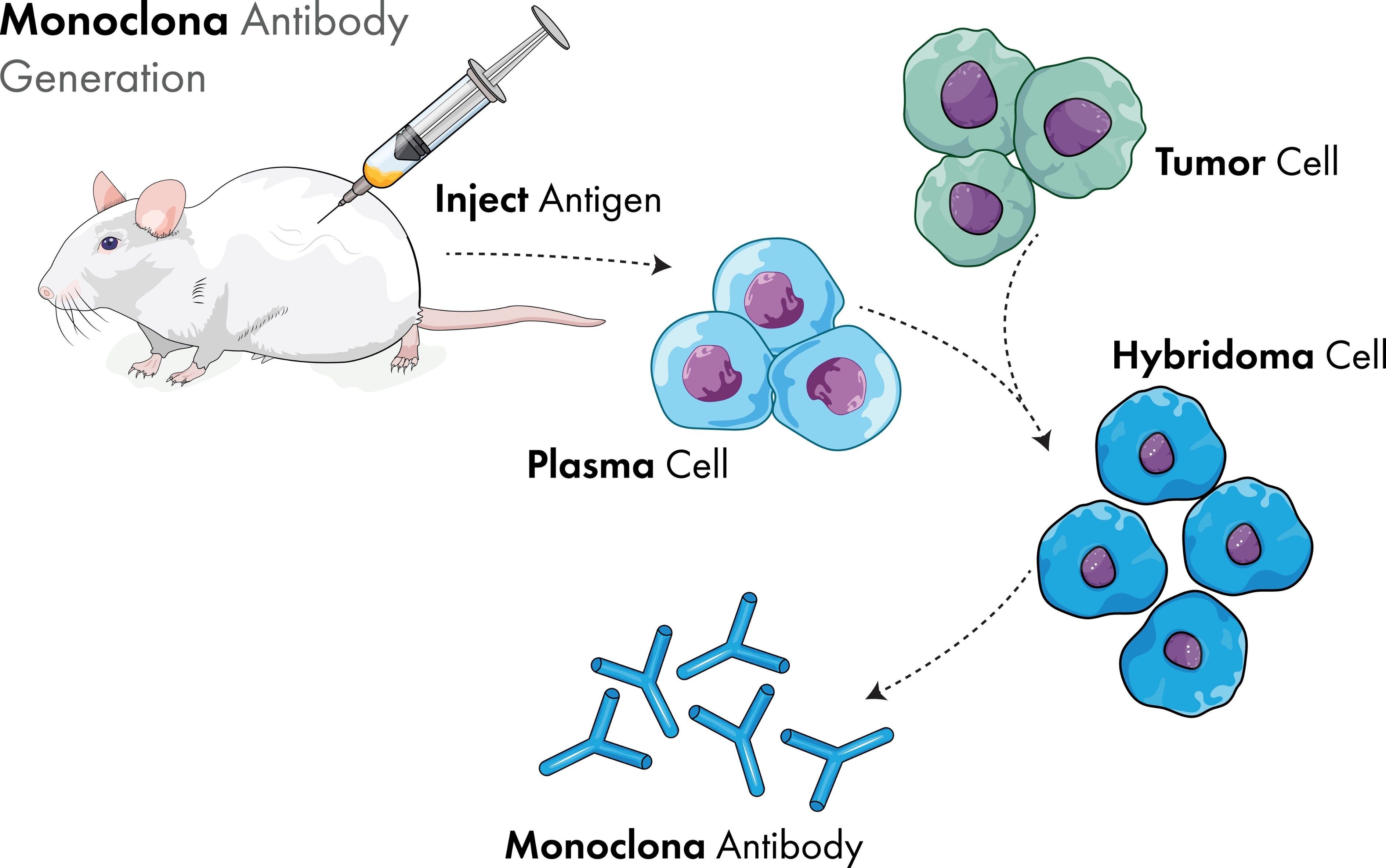
Hybridoma technology is also called monoclonal antibody technology. Its main process is to immunize animals first and inject specific antigens into mammals so that a large number of B lymphocytes that secrete specific antibodies against the antigens will be produced in the spleen. Since B lymphocytes are terminally undifferentiated cells, they will die after living for a period of time. Therefore, it is necessary to fuse B lymphocytes with myeloma cells that do not secrete antibodies but can proliferate and survive in vitro to produce hybridoma cells that have two cell characteristics and can secrete antibodies and proliferate indefinitely. After the fusion of two kinds of cells, five types of cells will be produced, which are unfused B lymphocytes, unfused myeloma cells, B lymphocyte-B lymphocyte fusion, myeloma cell-myeloma cell fusion, and desired hybridoma cells. The next step is to screen the hybridoma cells to remove the four types of cells that are not needed.
Fig. 1 Schematic diagram of the principle of hybridoma technology
The screening of hybridoma cells generally includes two steps. First, HAT medium is used for screening. HAT medium contains substances such as hypoxanthine (H), aminopterin (A), and thymidine nucleotide (T). Aminopterin can block the biosynthetic pathway of DNA, and myeloma cells cannot proliferate and die rapidly because of the blocked biosynthetic pathway and the lack of an emergency synthetic pathway. B lymphocytes generally die in about 10 days due to lack of ability to proliferate in vitro. Hybridoma cells have the ability to proliferate in vitro, and due to the presence of hypoxanthine and thymidine nucleotides, they can synthesize DNA through emergency pathways and grow normally in HAT medium without dying. Therefore, the fused cells are cultured in HAT medium, other fusion results will all die, and hybridoma cells are finally screened out.
Next, screen a single hybridoma cell, dilute the medium into a multi-well plate, so that each well contains only one hybridoma cell, and then produce from these single-cell clones, and finally secrete a hybrid cell line with a predetermined specific antibody for expanded culture. When screening a single hybridoma cell, the limiting dilution method or semi-solid medium method is usually used. For the limited dilution method, in order to comply with FDA declaration requirements, two rounds of limited dilution cloning (LDC) or one round of LDC combined with image proof are required to verify monoclonality. The limited dilution cloning method requires a lot of work and often requires the use of automated workstations for high-throughput hybridoma screening.
Antibody Library Technology
The technology of antibody libraries has progressed through three stages: initial combinatorial antibody library, phage display antibody library, and ribosome display antibody library. The initial combined antibody library is to clone the complete set of antibody light chain and heavy chain Fd fragment genes from lymphocytes by RT-PCR method, and construct them into expression vectors respectively, and infect E. coli to obtain light chain and heavy chain gene libraries. The light chain and heavy chain genes are then recombined at random into the same expression vector to create a combined antibody library.
The phage antibody library swiftly surpassed the combinatorial antibody library. It expresses and displays foreign polypeptides or proteins on the surface of phages and then obtains phages expressing specific peptides or proteins through panning and enrichment to obtain target antibodies. Its primary procedure is as follows:
- Construction of a phage display library: amplify the antibody gene library from B lymphocytes, insert it into the genome of the phage, fuse the antibody fragments with the phage capsid protein, and display them on the surface of the phage to form a phage display antibody library.
- Antibody library panning: incubate the phage library with the immobilized antigen, wash the unbound phage, and then elute the phage that specifically binds to the antigen. This process needs to be repeated 3–4 times to enrich for high-affinity phages. In the screening process, ELISA or WB methods are generally required for enrichment verification.
- Phage infection and screening: infect coli with high-affinity phages, and culture them on agar plates to select clones.
- Antibody verification: after screening the appropriate phage, perform sequencing to obtain the correct sequence for antibody expression. At this point, antibody affinity measurement and functional identification are required.
Phage display technology has many advantages. It achieves the unification of protein/polypeptide genotype and phenotype, establishes a direct physical connection between protein and its genetic gene, and can quickly screen to obtain the desired antibody. This approach can screen not just specific antibodies to general antigens, but also antibodies to non-immunogenic or hazardous antigens, and the resulting antibodies can be Fab, scFv, or other frequently utilized antibodies. The use of phage display technology to generate monoclonal antibodies is quick, simple, and efficient, and it may be utilized to generate humanized antibodies, which have a high usage value.
Single B Cell Antibody Technology
Single B cell antibody technology, a new generation of antibody development technology, can efficiently and swiftly extract antibodies from a single B cell, which is another milestone in antibody production technology after hybridoma and phage display. This technique differs in two ways that first, the step of immunizing mice with antigens is omitted, and second, it no longer relies on mouse B cells that must be hybridized with myeloma.
Its main procedure is to collect peripheral blood mononuclear cells from immunized animals or humans and then sort antigen-specific binding B cells. Then, using PCR amplification technology, a single B cell antibody variable region gene was acquired, followed by antibody expression via vector assembly, and ultimately, product verification. During this process, the isolation of single B cells is the most critical step in single B cell technology. At present, there are many techniques based on single cell separation, such as magnetic bead sorting, micro-engraving and ISAAC methods, flow cytometric sorting, and microfluidic sorting.
Flow cytometry is one of the most widely used single B cell techniques. It is based on the specific reaction of antigens and antibodies, using fluorescein-labeled antibodies to B cell surface molecules and labeled specific antigens to screen antigen-specific B cells through multicolor flow cytometry analysis and sorting. Flow cytometric sorting can directly sort a single antigen-specific B cell into a single well of a cell culture plate for subsequent antibody gene amplification and determination. Advantages of this technology: rapid and accurate separation of B cells, a large number, and simultaneous analysis of multiple parameters.
A single specific B cell is obtained by single cell isolation technology—the IgG heavy chain and light chain variable region genes are amplified from B cells by single cell PCR technology; sequence information is obtained by sequencing; and then vector construction, antibody expression, and verification are carried out by genetic engineering technology.
Creative Biolabs has a variety of high-throughput screening technologies and diversified identification and characterization methods that can help accelerate the development of antibodies.