Compared with traditional antibodies, single-domain antibodies (sdAbs) have small molecular weight, high hydrophilicity, and can be recombinantly expressed in vitro and mass-produced in E. coli, which can effectively avoid the difference between batches of traditional antibodies. Because of the above characteristics, single-domain antibodies have a series of advantages in new drug discovery and show great potential, including:
- The ability to bind to epitopes that cannot be bound by traditional antibodies
- Stronger target binding specificity
- Higher tissue penetration
- Higher stability
- Suitable for large-scale industrial production
- Easier to be modified and optimized
- Easier to be humanized
Based on these advantages of single-domain antibodies, more and more research institutions and pharmaceutical companies pay attention to and try to use single-domain antibodies in different fields. Different from preparing traditional monoclonal antibodies by hybridoma, the development of single-domain antibodies generally selects candidate single-domain antibodies by immunizing alpaca, constructing phage library and phage display technology, and then verifies whether these candidates can bind to antigens after single-domain antibody expression and purification.
Single-domain antibodies screening in immunized alpaca
The common method to obtain single-domain antibodies is to isolate B lymphocytes from the peripheral blood of the antigen immunized alpaca after antibodies are matured through the action of its immune system. Then RNA was extracted from B cells and reverse-transcribed to obtain cDNA. After PCR amplification with cDNA as substrate, a variety of single-domain antibody gene fragments were obtained, and then the gene fragments were linked to phagemids to construct a phage library. The candidate single-domain antibody was obtained from the alpaca antibody library after phage display and screening and then was verified. The whole process mainly includes alpaca immunization, phage library construction, and screening, expression, purification, and verification of antibodies.
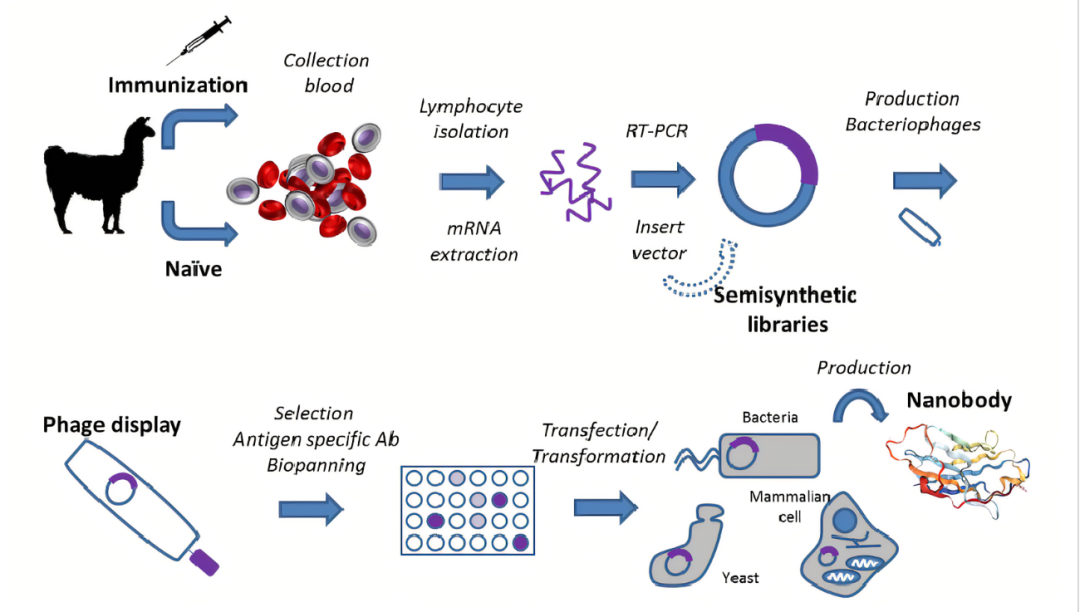
Flow chart of preparation of single-domain antibody
The process of alpaca immunization
- Antigen preparation
- Alpaca immunization
- Serum separation
- Blood collection for subsequent antibody titer detection
- Lymphocyte isolation
Immunization tips:
- The selection of alpaca and suitable antigens is the key to successful immunization. It’s advised to choose unimmunized alpacas that are healthy, strong, mentally fit, and of moderate size. The purity of immune antigens (> 90%) and their correct conformation are very important for screening suitable antibodies and subsequent applications.
- For lymphocyte isolation, timely cell isolation can effectively prevent hemolysis after blood collection to achieve the best isolation effect.
- The choice of the immune cycle can affect the immune effect. Experience shows that the immune interval of 1-2 weeks makes alpaca have a good immune response to most of the antigens.
The process of phage library construction
- RNA extraction
- Reverse transcription to obtain cDNA
- Antibody gene fragment amplification
- Cloning gene fragments into phage plasmids
- Plasmid TG1 transformation
- Amplification and purification of phage library
Phage library construction tips:
The capacity and diversity of the phage library are some of the important criteria for measuring library quality. The library with a larger capacity and better diversity is the effective guarantee for a successful screening of single-domain antibodies. The key factors affecting the library’s capacity and diversity include the degradation of RNA in the process of extracting RNA, the template and primer in the process of reverse transcription, the selection of primers and the amount of template in the process of amplification, the number of rounds of PCR amplification, the transformation efficiency of competent cells, the size of ligation system, and so on.
Process of antibody screening and identification
- Immunotubes coating
- Blocking
- Incubation of antigens and phages
- Cleaning
- Elution
- Titer detection of phage eluate
- Amplification of first round of phage eluate
- ELISA identification
Antibody screening tips:
- Appropriate antigen coating is related to the molecular weight, hydrophobic and hydrophilic properties, and structure of the antigen, as well as the choice of coating buffer and coating medium. Reasonable coating is the basis of successful antibody screening. If necessary, pre-experiments can be carried out to determine the conditions of coating, or the method of antigen binding magnetic beads can be selected for screening.
- The titer of bacteriophage should be determined after elution. After 2-4 rounds of antigen-coated panning, the degree of phage enrichment needs to be within a reasonable range.
Process of single-domain antibody expression and purification
The monoclonal strains screened by Phage-Elisa can be expressed, purified, and verified after single-domain antibody fragment DNA sequencing.
Single-domain antibodies can be expressed in E. coli, yeast, or mammalian cell lines. Because it contains only more than 100 amino acid residues, in order to verify whether it binds to the antigen, the plasmid can be rapidly expressed and purified in E. coli or yeast strain, or expressed and purified in both strains at the same time.
The advantage of the E. coli expression system is that it can induce expression quickly, but some single-domain antibodies can form inclusion bodies, and its intracellular reduction environment is not conducive to the formation of disulfide bonds in single-domain antibodies. The advantage of the Pichia pastoris expression system is that by the extracellular protein secretion mechanism of Pichia pastoris, the single-domain antibody can be purified directly from yeast fermentation supernatant without cell fragmentation. In addition, yeast rarely secretes miscellaneous proteins to the extracellular region so that single-domain antibodies in the fermentation supernatant are relatively pure and easy to be purified, and the extracellular oxidation environment is conducive to the formation of disulfide bond of single-domain antibodies, which is very important to the stability of single-domain antibodies.
