Creative Biolabs is one of the well-recognized experts who are professional in applying multiple technologies and advanced platform to assist a broad range of sdAb-related projects. As sdAbs present great potential in both therapeutic and diagnostic realm, our scientists are pleased to provide professional sdAb production and manufacture services to meet our clients’ specific requirements.
Custom Recombinant sdAb Production
As a part of our one-stop sdAb development package, scientists in Creative Biolabs have conducted a great deal of sdAb production with various requirements and modification. To generate the most suitable format of single domain antibodies, our scientists can take advantages of different constructs, expression systems, fusion tags, and labels that can contribute to your project purpose in the best manner. We can also provide high-quality production under GLP and GMP standard upon your request. Some popular construct formats include but are not limited to:
- sdAb-6×His via prokaryotic system.
- sdAb-Fc Fusion via eukaryotic system.
- sdAb-biotin via eukaryotic system.
- Bi-specific sdAb via eukaryotic system.
- sdAb-DsbC-6×His tag via prokaryotic system.
- sdAb-SUMO-6×His tag via prokaryotic system.
Depending on the special requirements of your project, our scientists will work closely with you to design and perform the best-fit procedure to meet your expectation in the quality, timeline, and budget.
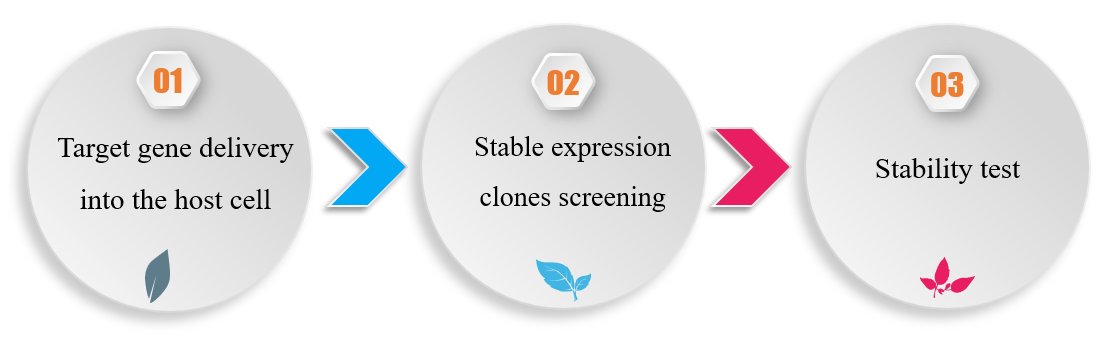
Cell Line Construction for Stable sdAb Manufacture
The construction of stable expression cell line with high yield is a crucial factor for the large-scale manufacture of single domain antibody. The whole generation cycle can be divided into three steps: target gene delivery, stable clones screening, and stability test. To customize high-quality stable cell lines, Creative Biolabs has built up a well-established system, which has contributed to a series of perfect stable cell lines construction for our clients’ manufactured demands.
Features
- High reproducibility and yield of over g/L
- One-stop service with full customization
- High-efficient cell line with high stability
Beyond that, our scientists are pleased to start the production procedure with provided in silico sdAb sequence from our clients. We can meet your general production demands via high-efficiency transient transfection or generate high-yield stable cell line for large-scale manufacture purpose. We can also provide a series of sdAb development and sdAb characterization solutions to assist your further project improvement.
Creative Biolabs is always dedicated to assisting our clients with the most satisfactory sdAb related solutions. If you are interested in our services, please feel free to contact us for more details.







