In the face of the threat of emerging pathogens, the rapid development of immunotherapy and the in-depth understanding of the diversity of antibody repertoire are beneficial. Single B cell sorting provides an efficient technology to achieve these goals.
Background
Monoclonal antibodies (mAbs) are produced by B cells against specific target antigens. In the human immune system, the antibody response is robust, highly specific, neutralizing, and self-tolerant. Using traditional hybridoma technology or transgenic mice to produce therapeutic human antibodies requires long-term immune procedures and screening, and clinical use of mouse antibodies may cause severe immunogenic responses (such as HAMA). Therefore, it is a very important and effective method to produce human mAbs by single B cell sorting technology in emergencies such as infectious diseases. This technique requires only a few cells so that potential mAbs can be isolated efficiently and quickly. In addition, single B cell clones retain the biologically-mediated pairing of heavy and light chains and maintain binding affinity.
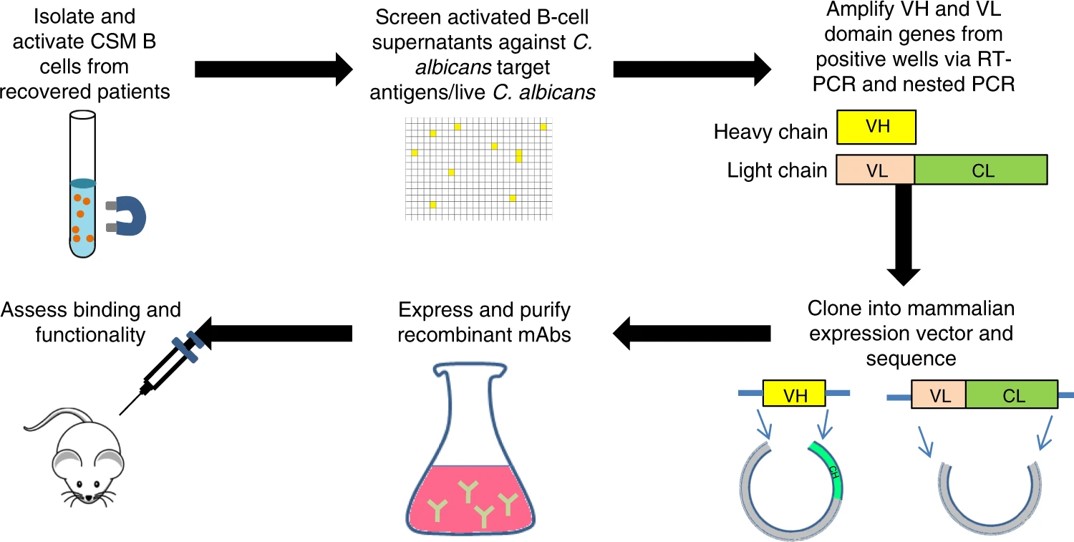 Fig.1 Generation of human mAbs from single B cells.1, 3
Fig.1 Generation of human mAbs from single B cells.1, 3
Cases: Single B Cell-derived Antibodies for Microbial Infection Treatment
Antibody memory is a hallmark of adaptive immunity, which can protect life from many pathogens. For bacterial, parasitic, viral, and other infectious diseases, the technology of producing antibodies by single B cell method has been widely used.
Among bacteria, Bacillus anthracis is a fatal pathogen that can cause severe anthrax disease in humans and has been used as a biological weapon. Although antibiotics can be used for the treatment of anthrax and as post-exposure prevention, the anti-anthrax protective antibody from a single human B cell will still be an important supplement to the treatment toolbox.
Different influenza viruses cause epidemics every year, and the influenza vaccine is the most effective measure to prevent seasonal influenza. Single B cell separation to produce effective and widely neutralized anti-influenza antibodies has become a popular work.
Studies have been conducted on the rapid isolation of Dengue neutralizing antibodies from human antigen-specific memory B cell cultures, and the characteristics of antigen-specific B cells in the peripheral blood of Dengue virus-immunized individuals have been reported. For rotavirus, perform a single B cell method to analyze the rotavirus antibody gene repertoire of VP6-specific B cells in the naive and memory B cell subpopulations, and generate rotavirus-specific human mAbs by sorting single B cells from the small intestinal mucosa. In addition, the human mAb against Zika virus NS1 produced by the single B cell method also showed a good therapeutic effect.
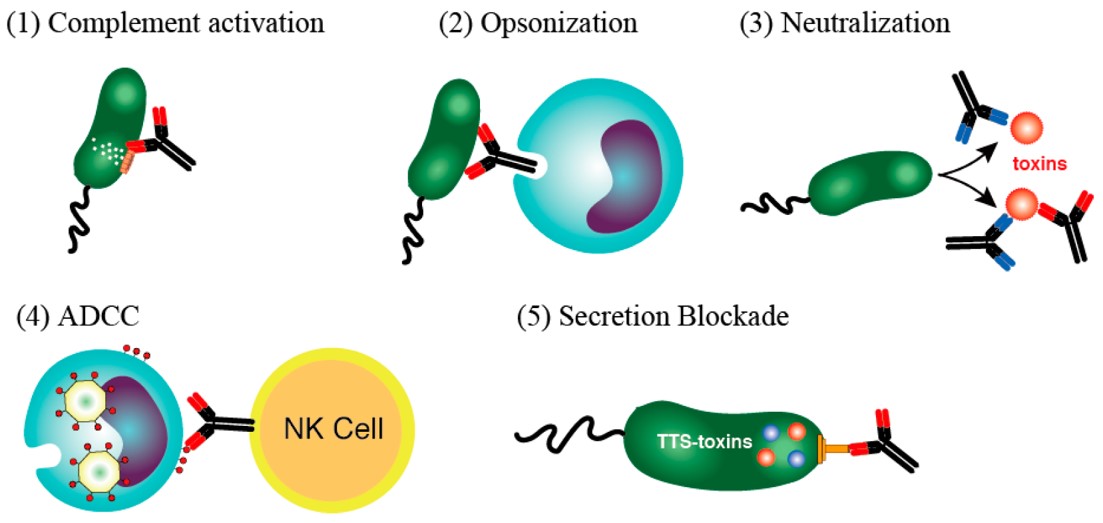 Fig.2 Mechanisms initiated by antibodies to destroy bacteria or toxins.2, 3
Fig.2 Mechanisms initiated by antibodies to destroy bacteria or toxins.2, 3
In the process of producing therapeutic mAbs by single B cell sorting technology, antigen labeling technique, the configuration of antigen sorting (such as monomer or dimer) and the design of primer sets are important factors for the successful generation of mAb. In Creative Biolabs, using the Native™ antibody discovery platform to recover mAbs from single B cells is a powerful tool. Combined with next-generation sequencing, new diagnostic and pharmacokinetic applications can be developed. For more detailed information, please feel free to contact us.
References
-
Rudkin, Fiona M., et al. "Single human B cell-derived monoclonal anti-Candida antibodies enhance phagocytosis and protect against disseminated candidiasis." Nature communications 9.1 (2018): 5288.
-
Sawa, Teiji, et al. "Immunoglobulin for treating bacterial infections: One more mechanism of action." Antibodies 8.4 (2019): 52.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Not For Clinical Use.
Related Sections:
ONLINE INQUIRY

 Fig.1 Generation of human mAbs from single B cells.1, 3
Fig.1 Generation of human mAbs from single B cells.1, 3
 Fig.2 Mechanisms initiated by antibodies to destroy bacteria or toxins.2, 3
Fig.2 Mechanisms initiated by antibodies to destroy bacteria or toxins.2, 3

