Having been committed to monoclonal antibody development for decades of years, Creative Biolabs provides a high-throughput B cell sorting platform, which is capable of screening a large number of B-cells in a time-saving manner.
Cell Isolation for B Cell Antibody Discovery
Since the first therapeutic monoclonal antibody (mAb) targeting the CD3 was approved by the Food and Drug Administration in 1986, antibody technology and engineering have flourished. Currently, more than 80 therapeutic mAbs have been approved and hundreds of mAbs have been evaluated in clinical trials all through the world. Most of the therapeutic mAbs on the market were produced by hybridoma, phage display, and transgenic mice technologies. To overcome the limitations of current antibody production methods such as inevitable immunogenicity and unsatisfactory affinity, single B cell antibody technology has been developed for isolating single antigen-specific B cells to retrieve Native™ antibodies with high in vivo affinity and lower/no immunogenicity.
For initiating Native™ antibody development, single B cells need to be isolated and identified. Based on the principles used, the existing techniques can be classified into two groups, B cell random isolation and antigen-specific B cell sorting.
Approaches for B Cell Random Isolation
Compared with single antigen-specific single B cell isolation, procedures for B cell random isolation are relatively simple, just directly picking B cells or plasma cells from samples. This random isolation is recommended for blood samples with high antibody titers, such as samples from vaccinators or infected patients. Methods for B cell random isolation mainly including but not limited to:
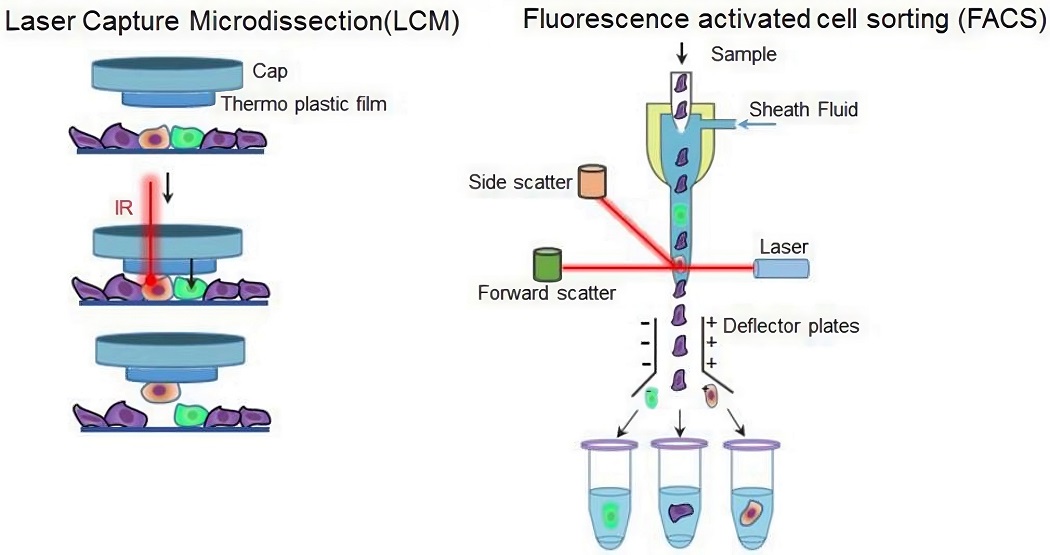 Fig.1 Techniques for single cell random isolation.1
Fig.1 Techniques for single cell random isolation.1
Micromanipulation technique is a convenient and simple method that manually selects and isolates single B cells under the high-power microscope by micromanipulators. The limitations of this micromanipulation are low isolation efficiency and the limited number of cell types isolated.
Laser capture microdissection is an automated technology that directly isolates specific cells of interest from solid samples (i.e., frozen or paraffin-embedded tissue sections) without destroying the tissue structure, which can be used for histologically pure B cell enrichment. The only limitation of this approach is the complexity of sample preparation.
Based on fluorescent agents and cell sorting instruments, FACS automatically sorts target cells according to the fluorescence signal on the cells. FACS is one of the most commonly used approaches, which is available for both random B cell isolation and antigen-specific B cell selection.
Limiting dilution for individual B cell isolation is performed by successive dilution of the cell suspension until the number of cells in a highly diluted sample may be as low as one single cell per aliquot. This limiting dilution is commonly applied for the production of monoclonal cells, which also is suitable for single B cell random isolation.
Creative Biolabs has been a trusted provider of top-notch research antibodies and related solutions worldwide. Based on our B cell sorting platform, we offer comprehensive Native™ monoclonal antibody development for various species. Please feel free to contact us and our experienced technicians will give you the most detailed answers to your questions.
Reference
-
Hu, Ping, et al. "Single cell isolation and analysis." Frontiers in cell and developmental biology 4 (2016): 116. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image.
For Research Use Only. Not For Clinical Use.
Related Sections:
ONLINE INQUIRY

 Fig.1 Techniques for single cell random isolation.1
Fig.1 Techniques for single cell random isolation.1

