Why Choose Custom iPSC-derived Cell Services?
At the heart of custom iPSC-derived cell services is the versatility of induced pluripotent stem cells (iPSCs). These cells hold the unique power to differentiate into virtually any immune cell type, while maintaining genomic stability, patient relevance, and limitless expansion potential. But transforming iPSCs into reliable, functional immune cells requires more than just standard protocols — it demands expert handling, iterative optimization, and deep biological insight. That's exactly what Creative Biolabs delivers.
Tailored to Fit Your Research
Industrial-Grade Scalability
Full Genetic Control
End-to-End Quality Assurance
Our scientific team combines 20+ years of immunology, stem cell biology, and therapeutic development experience. We understand how immune cells behave — and how to engineer them to behave exactly the way you want.
Service Portfolio
We specialize in developing highly functional immune cells from iPSC lines, tailored for applications across oncology, autoimmunity, infectious disease, and regenerative medicine. Explore our key offerings below.
Custom iPSC-derived Macrophage Service
Macrophages are central players in innate immunity, inflammation, and tissue remodeling. Our iPSC-derived macrophage services are ideal for:
- Modeling macrophage polarization (M1/M2) and cytokine profiles
- Studying phagocytic activity and inflammatory signaling
- Screening anti-inflammatory or immune-modulating drugs
- Developing macrophage-based cell therapies (e.g., CAR-M)
We offer M-CSF, GM-CSF, or IL-4 differentiated subtypes and can incorporate CRISPR edits, fluorescent tagging, or disease-specific mutations into your custom cell product.
Learn More →Custom iPSC-derived NK Cell Service
Natural killer (NK) cells are powerful effectors in anti-tumor immunity and antiviral defense. Our iPSC-derived NK cell services support:
- CAR-NK development with high cytotoxic activity
- Targeted tumor killing assays and ADCC studies
- Immunotherapeutic modeling for hematologic or solid malignancies
- Customizable phenotyping, receptor engineering, and in vivo testing
Whether you need standard NK-92-like profiles or uniquely engineered variants, we deliver cells with high CD56+CD3- expression and robust cytotoxic granule release.
Learn More →Custom iPSC-derived T Cell Service
T cells derived from iPSCs offer the rare combination of scalability and antigen specificity. This platform is particularly valuable for:
- CAR-T and TCR-T pipeline development
- Immune checkpoint functional screening
- Allogeneic T cell product design
- Modeling thymic development and immune tolerance
We support CD4+, CD8+, and regulatory T cell (Treg) differentiation from your selected iPSC line, including HLA editing and TCR knockout strategies.
Learn More →Our iPSC Customization Capabilities
We support custom differentiation into a wide range of cell types, including but not limited to:
| Category | Examples |
|---|---|
| Neural Cells | Dopaminergic neurons, motor neurons, sensory neurons, cortical neurons, astrocytes, oligodendrocytes, microglia, Schwann cells |
| Cardiac Cells | Cardiomyocytes, pacemaker cells, cardiac fibroblasts |
| Hepatic Cells | Hepatocytes, hepatic stellate cells |
| Renal Cells | Podocytes, renal tubular epithelial cells |
| Endothelial Cells | Blood-brain barrier (BBB) endothelial cells, vascular endothelial cells |
| Immune Cells | Macrophages, dendritic cells, NK cells, CD4+/CD8+ T cells, regulatory T cells |
If your desired cell type is not listed, reach out—we thrive on developing novel protocols.
Workflow of Custom iPSC-derived Cell
At Creative Biolabs, we deliver more than cells — we deliver confidence. Our custom iPSC-derived immune cell generation follows a stepwise, quality-assured workflow.
Project Consultation & Feasibility Analysis
We work closely with you to define your cell type, functional markers, application, and desired modifications (e.g., CRISPR, CAR, TCR edits).
iPSC Line Selection or Generation
Choose from our validated iPSC bank or provide your own. We also offer reprogramming from PBMCs, fibroblasts, or cord blood.
Custom Differentiation & Cell Expansion
Our team optimizes differentiation protocols (cytokine cocktails, co-culture, feeder-free systems) to achieve high-yield, functionally mature cells.
Functional Validation
Each cell product undergoes rigorous phenotypic and functional QC — flow cytometry, cytotoxicity assays, cytokine release, phagocytosis, etc.
Delivery & Post-project Support
Receive cryopreserved or fresh cells with full documentation. Follow-up consultations are always available to maximize application success.
Application Areas of Custom iPSC-derived Cell
Our custom iPSC-derived cell services are designed with translational goals in mind. These immune cells are ideal for the following studies.
| Application Area | Use Case Examples |
|---|---|
| Cancer Immunotherapy | CAR-T/CAR-NK development, cytotoxicity testing, checkpoint blockade studies |
| Autoimmunity Research | Modeling inflammatory responses, antigen-specific tolerance, cytokine secretion |
| Infectious Disease | Host-pathogen interaction models, antiviral immunity studies |
| Tissue Engineering | Co-culture with organoids, tissue remodeling, inflammatory microenvironment testing |
| Drug Screening | Small-molecule modulation, antibody screening, cell-based assays |
Case Studies of iPSC-derived Cell
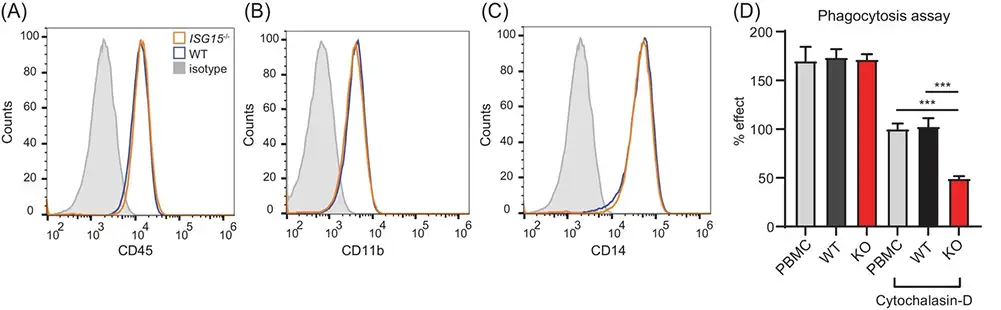
Case 1: iPSC-derived macrophages as a model to study the cellular phenotype of ISG15 deficiency and identify novel treatments
Waqas S F H et al. established a cellular disease model that encompasses cell types associated with systemic inflammation (iPSC-derived macrophages and vascular endothelial cells) and skin pathology. iPSCs were maintained on mouse embryonic fibroblasts, which were used as feeder cells or under feeder-free conditions on Geltrex-coated tissue culture flasks in E8 medium. For differentiation toward macrophages embryoid body (EB) formation was induced. EBs will start producing macrophages for up to 3–4 months, but the production rate will decrease with time. Macrophages were validated phenotypically and functionally in that they expressed CD45, CD11b, and CD14. In addition, they demonstrated phagocytic activity similar to M2-type macrophages derived from human PBMC.
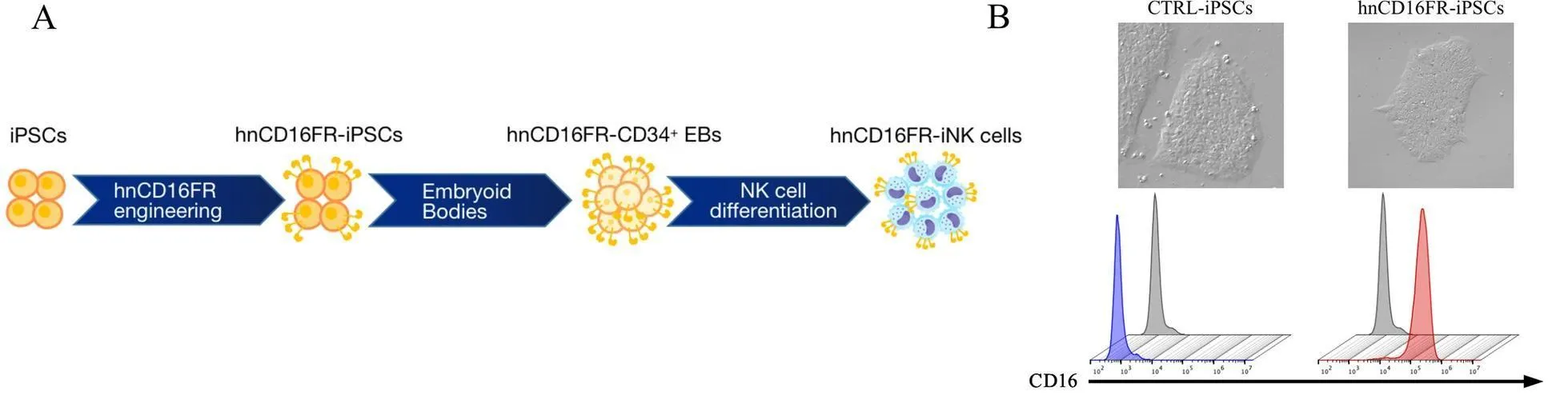
Case 2: FR constructs were transduced into human iPSC-derived NK cells
Meng, Fanyi, et al. designed the hnCD16 Fusion Receptor (FR) constructs. FR constructs were transduced into human iPSC-derived NK (iNK) cells and effective FR constructs were screened. For the generation of iPSCs, PBMCs were transduced using Sendai viruses encoding human OCT4, SOX2, KLF4, and c-MYC genes. To maintain stable hnCD16FR expression, they transduced human iPSCs with Control (eGFP) and hnCD16FR lentivirus and sorted positive cells for clonal expansion. The hiPSCs were differentiated first into hematopoietic stem/progenitor cells and then into NK cells.
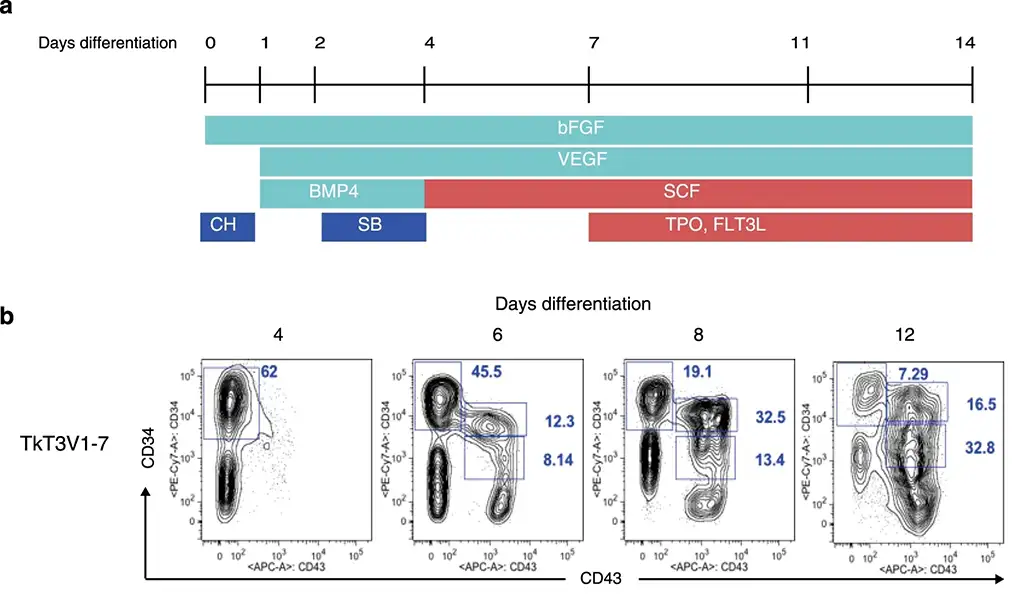
Case 3: A highly efficient and scalable method for T-cell generation from human iPSCs
Iriguchi, Shoichi, et al. reported the development of a highly efficient and scalable method for T-cell generation from human iPSCs. This system allows to generate T-cells from iPSCs derived from T-cell clones and T-cell receptor (TCR)-engineered iPSCs in the absence of feeder layers in all stages of differentiation. They divided the whole process into four steps and optimized each step, which includes induction of HPCs with lymphoid potentials from iPSCs, differentiation of HPCs into CD4+CD8αβ+ double-positive (DP) cells, maturation of DP-cells into CD8αβ+ single-positive (SP) T-cells, and finally proliferation of CD8αβ SP T-cells.
References
- Waqas, Syed Fakhar‐ul‐Hassnain, et al. "ISG15 deficiency features a complex cellular phenotype that responds to treatment with itaconate and derivatives." Clinical and translational medicine 12.7 (2022): e931. https://doi.org/10.1002/ctm2.931
- Meng, Fanyi, et al. "Leveraging CD16 fusion receptors to remodel the immune response for enhancing anti-tumor immunotherapy in iPSC-derived NK cells." Journal of Hematology & Oncology 16.1 (2023): 62. https://doi.org/10.1186/s13045-023-01455-z
- Iriguchi, Shoichi, et al. "A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy." Nature communications 12.1 (2021): 430. https://doi.org/10.1038/s41467-020-20658-3
- Distributed under Open Access license CC BY 4.0, without modification.
What Our Clients Say
“We requested a batch of gene-edited iPSC-derived CD8+T cells with specific HLA knockout, and they walked us through every step — from CRISPR design to delivery. QC data was publication-ready, and the T cells performed beautifully in our tumor killing assays.”
— Group Leader, Translational Immunology, Academic Research Hospital
“We were on a tight timeline to screen checkpoint inhibitors in a macrophage-T cell co-culture system. Creative Biolabs rapidly supplied both iPSC-derived M1 macrophages and functional CD4+T cells, fully characterized and assay-ready. Their responsiveness helped us stay on schedule.”
— Principal Investigator, Immunopharmacology
“Our company needed GMP-compatible iPSC-derived NK cells for an IND-enabling study. Creative Biolabs provided a clear manufacturing roadmap, adjusted culture conditions to meet regulatory needs.”
— Lead Scientist, US Clinical-stage Biotech
Get Started with Confidence














1. Contact Us
via the Inquiry Form or Email
2. Define Your Needs
Cell Type, Function, Quantity, Modifications
3. Kickstart the Project
Our Expert Team Guiding Every Step
Podcast
Stem Cells in a New Era of Cell-based Therapies
Stem Cell Therapy Podcast Series
FAQ about Custom iPSC-derived Cell Services
We already have iPSC lines in our company. Can they be used directly for the customization service?
Of course you can. We support the process of customization service for customer's own iPSC lines. Before starting the project, we will conduct a comprehensive quality assessment of your iPSC, including morphological examination, cell activity test, karyotyping and immunophenotyping of conventional surface markers.
If your cells have passed the basic certification, we will directly use them for directed differentiation; if problems are found, we can also assist you in cell recovery, expansion or reprogramming treatment to ensure stable and reliable quality of basic cells for the whole project.
Do you have experience in the targeted induction of iPSC to generate Tregs?
Can you provide a functional comparison of the three iPSC-derived cells or a model recommendation?
Of course, our team of scientific advisors can recommend the most appropriate cell type for your project purpose (e.g. anti-tumor, inflammation modulation, anti-virus, etc.).
- iPSC-Macrophage: Suitable for inflammation models, drug phagocytosis testing, M1/M2 polarization studies.
- iPSC-NK Cell: Advantage is fast, non-MHC-dependent killing, suitable for CAR-NK research, anti-tumor screening model.
- iPSC-T Cell: Suitable for antigen-specific studies, immune response modeling, CAR-T or TCR-T development.