Creative Biolabs is able to provide state-of-art custom services for any inducible pluripotent stem cell (iPSC)-derived T cells project with qualitative measurements. Our scientists specialized in stem cell therapy studies will work with you to develop a most appropriate strategy that will offer the most meaningful data for your research. We guarantee the finest results for our customers all over the world.
iPSC technology has provided a new opportunity for generating a number of cell products applied in cell immunotherapy. Previous studies have illustrated that iPSCs can constantly produce T lymphocytes and can be easily cultured in vitro to trigger immune responses. Meanwhile, genetic engineering of iPSCs-derived T cells can emerge specific properties into the development of cell therapy to treat plenty of malignant diseases, such as tumors. Furthermore, recent researchers have revealed that T cells derived from iPSCs play an important role in maintaining the structural stability of T cell receptors (TCRs) during in vitro differentiation. As a result, iPSCs-derived T cells with the desired characteristics can be broadly used for generating therapeutic T cells in disease treatment. For example, iPSCs-derived T cells are capable of recognizing tumor antigens, including LMP2, WT-1, and MART-1, preventing the TCR rearrangement and keeping the antigen specificity in the cell therapy process.
The potential role of iPSCs in cell therapy has been proved in the past few years. Up to now, iPSCs have become a valuable source of iPSC-derived T cells in treating a wide variety of human diseases, especially for various cancers. Therefore, Creative Biolabs has developed a series of custom assays to generate different iPSC-derived T cells, and identified their phenotypes or functions in xenograft models. For the differentiation methods, we have established detailed protocols to describe the process of iPSC-derived T cell development. In general, iPSCs are commonly induced to form mesoderm and then to produce hemogenic endothelium. Hemogenic endothelium further differentiates into hematopoietic stem and progenitor cells (HSPC). The final step is to obtain and test the functionality of mature CD8 or CD4 SP T cells. In addition, in order to avoid alloreactivity of iPSC-derived T cells, we have equipped with many advanced technologies for assessing TCR genetic modifications in the iPSCs.
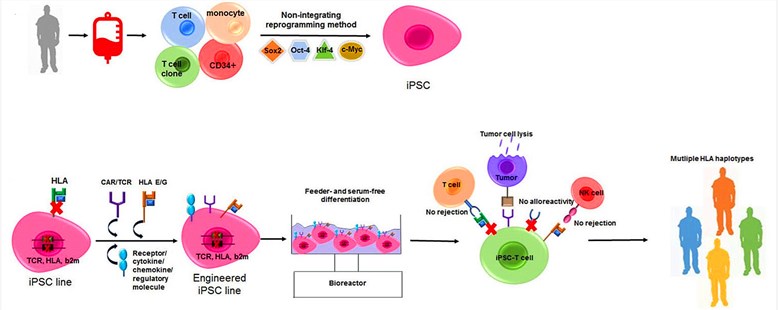 Fig.1 Generation of iPSC-derived off-the-shelf tumor-specific T cells.1
Fig.1 Generation of iPSC-derived off-the-shelf tumor-specific T cells.1
Our service leverages the power of iPSCs to differentiate them into functional T cells, providing researchers with a renewable and scalable source of T cells for various applications.
Here is an overview of the process involved in our custom iPSC-derived T cell service:
Our service provides researchers with a reliable and customizable platform to generate high-quality T cells for a wide range of applications in biotechnology research.
As a well-established company in the bio-industry, we offer custom stem cell therapy services based on iPSC technology. Guaranteed to perform project within the specified time frame, and the quality of iPSC-derived T cell product is always maintained to the end. Other features:
By focusing on quality, scientific expertise, flexibility, and responsiveness, Creative Biolabs is dedicated to providing a flexible and expedient iPSC-derived T cell solutions to meet global regulatory standards. Experienced teams of researchers and technicians have enabled our company to undertake any research with the highest standards and solid knowledge that span a wide range of stem cell therapy development and therapeutic areas. For additional details on our iPSC-based cell therapy services, please for free to contact us or send us an inquiry.
Below are the findings presented in the article related to ipsc-derived T cells.
Researchers have generated dual antigen receptor (DR) T cells from iPSC to attenuate tumor antigen escape. These cells were modified to express a chimeric antigen receptor (CAR) directed against antigenic cell surface latent membrane protein 1 (LMP1) and a T-cell receptor directed against cell surface latent membrane protein 2 (LMP2), which binds to human leukocyte antigen A24 for the treatment of treatment-naïve Epstein-Barr virus-associated lymphoma.
In their study, they demonstrated that the constructed iPSC-derived DR T cells could recognize LMP1 antigen via CAR and LMP2 antigen via natural TCR. In treatment, these cells were superior to peripheral blood-derived LMP1-CART. They showed 100% survival and potently eliminated ENKL not only once. They also generated cells that recognized CD19 antigen via CAR and LMP2 antigen via the natural TCR to confirm reproducibility of results. Both types of iPSC-derived T cells had potent and long-lasting effects on EBV-driven tumors.
Reference
For Research Use Only. Not For Clinical Use.