What Is the Teratoma Formation Assay?
The teratoma assay involves transplanting iPSCs into immunocompromised mice, where they spontaneously differentiate into a benign tumor (teratoma) containing disordered but functional tissues derived from all three germ layers. This process mirrors early embryonic development and remains the only definitive in vivo test for human iPSC pluripotency. Key steps include:
- Cell Preparation: iPSCs were harvested using EDTA or trypsin-based isolation and resuspended to enhance transplantation.
- Transplantation: Cells are injected into areas such as the testicular capsule or intestinal muscle (for surgical accessibility).
- Monitoring: Teratomas typically form within 6–12 weeks, with iPSCs often showing faster kinetics (as short as 4 weeks) compared to embryonic stem cells.
- Analysis: Histological examination of the removed teratoma was performed to confirm the rules of multi-lineage differentiation and malignant components.
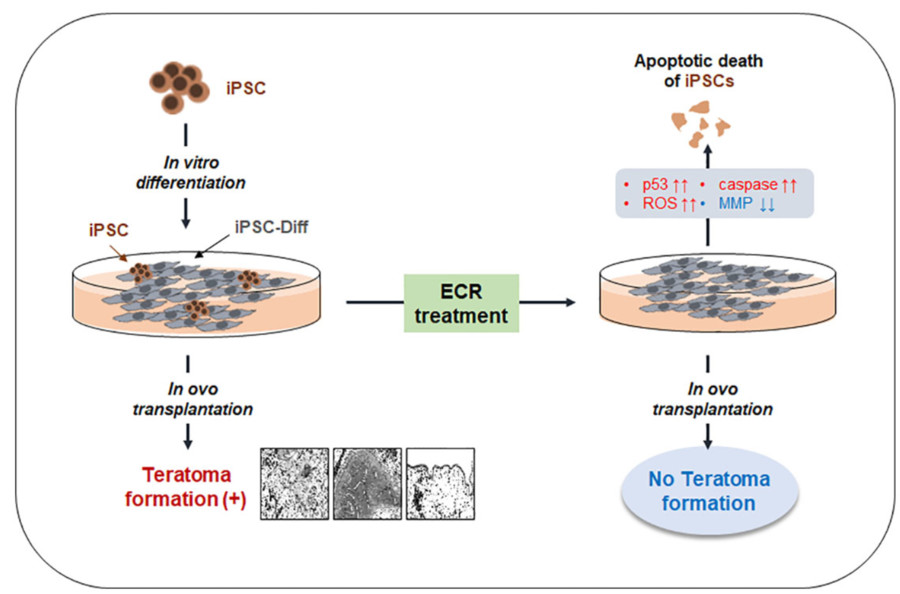 Fig. 1 A flowchart depicting the process by which iPSCs undergo apoptotic cell death and inhibit the formation of teratomas.1,4.
Fig. 1 A flowchart depicting the process by which iPSCs undergo apoptotic cell death and inhibit the formation of teratomas.1,4.
Service Advantages
- Unbiased Pluripotency Assessment: Unlike in vitro assays, teratoma formation provides a holistic evaluation of differentiation potential within a complex biological environment, eliminating artifacts from culture conditions.
- Standardized Protocols: We employ optimized injection sites and immunodeficient mouse models to achieve 95–100% teratoma formation efficiency with reproducible kinetics.
- Advanced Histopathological Analysis: Teratomas are sectioned, stained with hematoxylin and eosin (H&E), and evaluated by certified pathologist.
- Tumorigenicity Risk Screening: Early detection of residual undifferentiated cells or malignant transformation is critical for the preclinical safety of iPSC-derived therapies.
Technical Workflow of Teratoma Formation Assay Service for iPSC
| Process | Description |
|---|---|
| iPSC Preparation & Quality Control | Detection of pluripotency markers of iPSC by flow cytometry. Respond 1-2 million cells in a mixture for optimal cell survival and injection. |
| Animal Model Selection | Utilize NSG mice for superior engraftment and lifespan. Administer analgesics and antibiotics pre-/post-surgery to ensure welfare. |
| Surgical Transplantation | Testis capsule injection: Expose testes via abdominal incision; inject cells avoiding major vessels. Intramuscular injection: Deliver cells into the gastrocnemius muscle using insulin syringes for minimal invasiveness. |
| Teratoma Monitoring & Harvesting | Palpate weekly for tumor growth; sacrifice mice when tumors reach ~1 cm³. |
| Histopathology & Reporting | Fix teratomas in 4% paraformaldehyde, embed in paraffin, and section for H&E staining. Provide detailed reports with images confirming trilineage differentiation and tumor morphology. |
Cutting-Edge Technologies Driving Our Services
To ensure gold-standard validation of iPSC pluripotency and biosafety, our teratoma formation assay service integrates the following advanced technologies and innovative methodologies.
- High-Sensitivity In Vivo Imaging
- Alternative Animal Models for Ethics & Efficiency
- Standardized Protocols for Reproducibility
- Multi-Omics Quality Control
- Quantitative Sensitivity Profiling
- Integrated Biosafety and Pluripotency Reporting
Applications of Teratoma Assay Data
- Pluripotency Validation: Critical to identifying new iPSC lines or confirming stability after genetic modification.
- Safety Profiling: Identifying tumorigenic risks of iPSC-derived products for cell therapy applications.
- Drug Development: Assessing the safety of stem cell therapy.
- Disease Modeling: Study aberrant differentiation patterns in iPSCs from patients with developmental disorders.
Why Choose Our Teratoma Assay Service?
| Feature | Our Service vs. In-House Efforts |
|---|---|
| Standardization | Strict SOPs and reduce inter-lab variability |
| Efficiency | High teratoma formation rates with shortened latency |
| Expert Analysis | Certified pathologists provide detailed germ layer quantification and risk assessment |
| Speed | Complete assay in 8–12 weeks vs. typical 12–16 weeks for self-operated assays |
Published Data
In this study, they identified CD82+ERBB3+NGFR+cells from human iPSC-derived teratomas with long-term regenerative capacity in NSG-mdx4Cv mice. MyHC isoform analysis showed progressive maturation from embryonic to slow fiber types over time. These progenitors-maintained engraftment potential even after cryopreservation. The study highlights the long-term regenerative ability of teratoma-derived human skeletal myogenic progenitors.
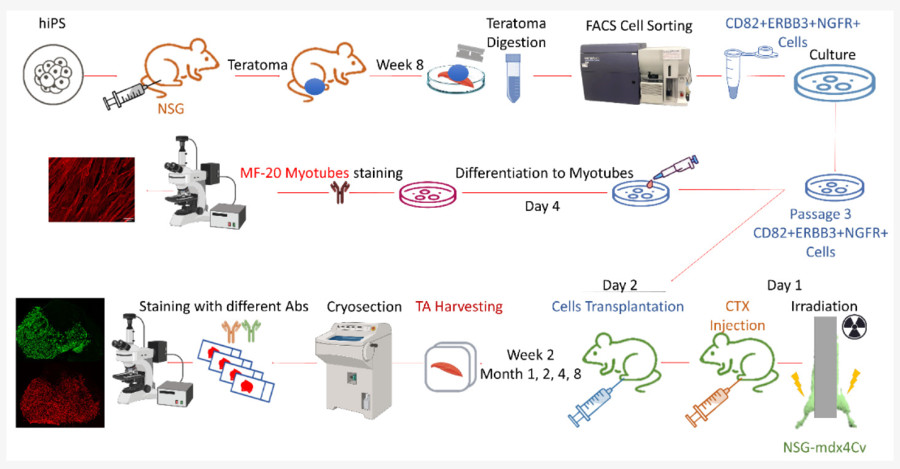 Fig. 2 The main steps of injecting hiPSCs into NSG mice TA muscles and removing teratomas after two months.2,4
Fig. 2 The main steps of injecting hiPSCs into NSG mice TA muscles and removing teratomas after two months.2,4
In this study, researchers demonstrated that MSC can indeed be isolated from the small, concentrated MSC mass within iPSC-generated teratoma. These iPSC-derived MSCs exhibited similar cellular characteristics to traditional MSCs. Adult MSCs, such as adipose-derived stem cells (ASC) and bone marrow-derived stem cells (BM-MSC). Overall, these findings strongly suggest that fetal tumors produced by iPSC may develop concentrations.
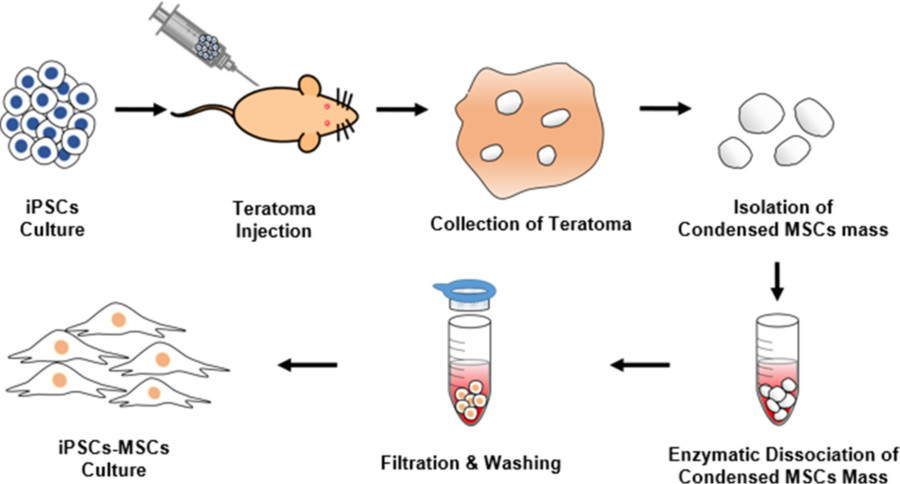 Fig. 3 Schematic diagram depicting the entire isolation process of iPSCs-derived MSCs from teratomas.3,4
Fig. 3 Schematic diagram depicting the entire isolation process of iPSCs-derived MSCs from teratomas.3,4
What Our Clients Say
"Their teratoma assay provided unambiguous evidence of pluripotency for our novel iPSC line. The comprehensive histology report revealed robust differentiation into all three germ layers, which was crucial for our submission."
— Dr. Emily Reed, Regenerative Medicine Program
"We avoided months of optimization by outsourcing teratoma assays. Their use of non-invasive imaging allowed us to track teratoma growth in real-time, reducing costs."
— Prof. James Lee, Drug Safety Institute
FAQs
Q: How many cells are required per injection?
A: We recommend 1–2 million cells per injection to ensure consistent teratoma formation.
Q: Can assays be performed with iPSCs derived using non-integrating methods (e.g., mRNA)?
A: Yes. Our service is compatible with footprint-free iPSCs and includes genomic screening to rule of residual vector integration.
Q: Do you provide cryopreserved teratoma samples for further analysis?
A: Yes. Customers can request that frozen sections be subjected to immunohistochemistry or RNA sequencing to verify specific lineages.
Take the Next Step with Creative Biolabs














1. Contact Us
via the Inquiry Form or Email
2. Define Your Needs
Cell Type, Function, Quantity, Modifications
3. Kickstart the Project
Our Expert Team Guiding Every Step
Ensure the biological relevance and safety of your iPSC lines with our end-to-end teratoma formation assay services. Our team provides customized project design, rigorous execution, and actionable data to advance your research or therapy development.
Inquire now for a detailed quote and project timeline.
References
- Kim, Aeyung, et al. "An Ethanol Extract of Coptidis rhizoma Induces Apoptotic Cell Death in Induced Pluripotent Stem Cells and Suppresses Teratoma Formation." Nutrients 15.10 (2023): 2364. https://doi.org/10.3390/nu15102364
- Khosrowpour, Zahra, et al. "Long-Term Engraftment and Satellite Cell Expansion from Human PSC Teratoma-Derived Myogenic Progenitors." Cells 14.15 (2025): 1150. https://doi.org/10.3390/cells14151150
- Kim, Jiseong, et al. "Therapeutic potential of mesenchymal stem cells from human iPSC‐derived teratomas for osteochondral defect regeneration." Bioengineering & Translational Medicine 9.2 (2024): e10629. https://doi.org/10.1002/btm2.10629
- Distributed under Open Access license CC BY 4.0, without modification.
Created September 2025

