Chimeric antigen receptor (CAR) T cells have been remarkably successful in the treatment of relapsed hematologic malignancies, such as acute lymphoblastic leukemia (ALL), non-Hodgkin’s lymphoma (NHL), and multiple myeloma (MM). CD19 and B-cell maturation antigen (BCMA) are two of the most successful CAR targets with good response rates. The total effective rate (ORR) of approved CAR-T drugs targeting CD19 reaches more than 70%, while idecabtagene vicleucel and ciltacabtagene autoleucel targeting BCMA reach 73% and 97%, respectively. However, at least 50% of patients treated with CAR-T cells still experience relapse or progression.
The main mechanisms of relapse after CAR-T cell therapy are the limited persistence of CAR-T cells, CAR-T cell functional inhibition, and antigen escape. To minimize the risk of relapse due to target escape, strategies for dual-target CAR-T to recognize more than one tumor-associated antigen are being actively explored in clinical trials. This strategy can be achieved by using two hybrid CAR-T cells with different antigen binding specificities or a single CAR-T cell capable of targeting two different antigens.
Currently, there are at least three combinations of antigens for dual-target CAR-T cell therapy in preclinical models and clinical trials for hematologic malignancies: CD19/CD20, CD19/CD22, and BCMA/CD38. Recently, dual-target CAR-T has been increasingly used in the clinic, and from the comprehensive clinical data disclosed, dual-target CAR-T shows extremely promising applications.
Common Dual-CAR Strategies
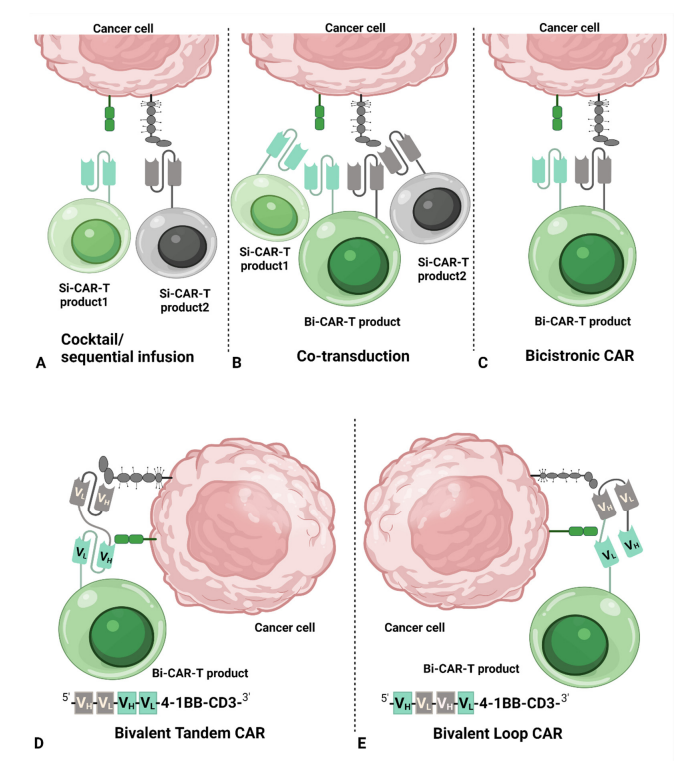
The main CAR structures used for dual-target CAR-T cell therapy include cocktail/sequential infusion, co-transduction, bicistronic CAR, bivalent tandem CAR, and bivalent loop CAR.
Cocktail/sequential infusion means two separate CAR-T cell products can be generated by transducing T cells with two different vectors, respectively. The two separate CAR-T cells are then mixed together in a 1:1 ratio and infused simultaneously or in a sequential infusion.
Another approach can be generated by co-transduction of T cells with two separate vectors, each encoding a separate CAR structure. Two separate single-target CAR-T cells and one dual-target CAR-T cell are included in the final hybrid product.
A bicistronic CAR-T cell is a bicis-transducible vector introduced into a T cell to generate two different CARs in the same T cell, where each CAR targets a different antigen-binding domain.
Bivalent tandem CAR-T cells are produced by introducing a bivalent vector into a T cell to produce a dual structural domain. By arranging the light chain variable domain (VL) and the heavy chain variable domain (VH) of a single chain antibody fragment (scFv) in a different order, two different structures can be formed: tandem and loop. Tandem structures are formed by directly connecting the VL-VH of one single-chain antibody to the VL-VH of another single-chain antibody, while loop structures are formed by sandwiching the VL-VH of one single-chain antibody between the VH-VL of another single-chain antibody.
Clinical Efficacy of Dual-Target CAR-T
The advantage of dual-target CAR-T cell therapy over single-target CAR-T cell therapy is its ability to reduce antigen escape from tumor cells. Clinical studies of single-target CAR-T cell therapy have shown over 90% complete response (CR), with little room for improvement in the initial response. Therefore, the expectation for dual-target CAR-T cell therapy is not only to improve the durability of response, but also to recreate response in patients who have relapsed or refractory after treatment with single-target CAR-T cells.
In a few studies, dual-target CAR-T cell therapy appears to have demonstrated superior Diagnostic Odds Ratio (DOR) and Overall Survival (OS) over single-target CAR-T cells. For example, OS in patients treated with CD19 single-target CAR-T cells was close to that of patients treated with CD22 single-target CAR-T cells, but shorter than that of patients treated with CD19/CD22 dual-target CAR-T cells. In non-Hodgkin’s lymphoma, the percentages of Progression-Free Survival (PFS) and OS were higher for CD19/CD20 dual-target CAR-T cell therapy than for CD19 single-target CAR-T cells.
The design strategy of dual-target CAR-T with CD19/CD20 or CD19/CD22 is based on the assumption that targeting CD20 or CD22 will facilitate the reduction of antigenic escape from CD19. Disclosed data suggest that single-target CAR-T cell therapy or dual-target CAR-T cell therapy targeting CD22 or CD20 can result in CR in patients with CD19 escape. In particular, seven patients who had previously received single-target CAR-T cell therapy for CD19, five of whom achieved CR after dual-target CAR-T cell therapy. Therefore, clinical data support CD19/CD20 or CD19/CD22 dual-target CAR-T cell therapy for relapsed patients who are resistant to CD19-CAR-T cell therapy.
In addition, in multiple myeloma, the combination of BCMA-CAR and a second CAR is still being explored. Dual-target CAR-T cell therapy with BCMA/CD19 and BCMA/CD38 is already in the clinic, while dual-target CAR-T cell therapy with BCMA/CS1 (SLAMF7) and BCMA/GPRC5D is about to enter the clinic. BCMA/CD19 dual-target CAR-T cell therapy showed encouraging efficacy in a small group of patients, with an ORR of 100% in five patients, only one grade 3 cytokine release syndrome (CRS) occurring, and no neurotoxicity (NT) occurring. In addition, BCMA/CD38 dual-target CAR-T cells also showed promising results—after treatment, five of the eight stringent complete response (sCR) patients remained sCR at a median follow-up of 9 months, with a 9-month PFS of 75%.
Safety Of Dual-target CAR-T Cells
Theoretically, dual-target CAR- T cell therapy is stimulated by two antigens, which raises the question of whether patients will have stronger CAR-T cell activation than single-target CAR-T cells, resulting in a higher incidence of adverse events than single-target CAR-T cells?
Available data suggest that the incidence of grade 1-2 CRS is similar between single-target CAR-T cell and dual-target CAR-T cell therapy. Among the available reported studies, grade 3-4 CRS was not reported in two of nine clinical trials (22.2%) of single-target CAR-T cell therapy, while grade 3-4 CRS was not reported in three of eight clinical trials (37.5%) of dual-target CAR-T cells. In addition, grade 3-4 CRS was not reported in 42.9% (3/7) of single-target CAR-T cell clinical trials neurotoxicity (NT), while 50% (4/8) of dual-target CAR-T cells did not report grade 3-4 NT.
A simple comparison shows that dual-target CAR-T cell therapy did not produce more severe CRS and NT, but rather lower, compared with single-target CAR-T cell therapy. Considering the sample size, the different clinical indications and the management of CRS and NT in the early phase of CAR-T cell therapy, it seems that more studies are needed to confirm this conclusion.
Summary
Although CAR-T cell therapy has achieved excellent results in hematologic malignancies, the problem of relapse remains critical. To overcome the tumor immune evasion of CAR-T cell therapy, multi-target CAR-T cell therapy has become a hot topic of current research. The efficacy of dual-target CAR-T cell therapy has been demonstrated in some clinical trials, and the safety seems to be superior to that of single-target CAR-T cells, with a lower incidence of both severe CRS and NT.
However, further optimization of CAR target selection and CAR structure is still needed to achieve 1+1>2 in dual-target CAR-T cell therapy. In conclusion, dual-target CAR-T cell therapy is still at an early stage of development and needs to be supported by more preclinical and clinical trial data, and it is believed that it will play a unique and critical role in tumor immunotherapy in the future.
Reference
1. Xie, Bailu, et al. “Current status and perspectives of dual-targeting chimeric antigen receptor T-cell therapy for the treatment of hematological malignancies.” Cancers 14.13 (2022): 3230.