Since 2017, when chimeric antigen receptor-T cells (CAR-T) were approved by the US Food and Drug Administration (FDA) as the first modified cell for the treatment of leukemia, five similar products have been approved and more than 20,000 people have received this treatment-changing immunotherapy.
CAR-T cells are designed to carry synthetic transmembrane receptor molecules, CARs, using the extracellular domain of the CAR to bind to antigens on cancer cells, while the endodomain of the CAR responds by initiating a powerful program of tumor cell destruction. However, not all patients respond equally well to CAR-T cell therapies, and cancer immunologists have been trying to figure out what makes them work well or fail.
Despite initial insights into the differences between T cells from cancer patients and those from healthy people, these insights have not been taken into account in the manufacturing process of CAR-T cells. All processes utilize similar T cell-specific agonists and common immunostimulatory cytokines for stimulation to produce infusion-ready CAR-T cell products, without consideration of changes in the original T cell phenotype.
Now, in a new study, Dr. David Mooney’s team at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Dr. Catherine Wu’s team at the Dana-Farber Cancer Institute have demonstrated that personalized stimulation of CAR-T cells during the manufacturing process can greatly enhance the consistency and potency of the resulting CAR-T cell products. The findings were recently published in Nature Communications in a paper titled “Enhancing CAR-T cell functionality in a patient-specific manner”.
Using an artificial antigen-presenting cell mimicking scaffold (APC-ms), the authors were able to fine-tune the level of T-cell stimulation to match the phenotype of T cells obtained from leukemia patients and significantly enhance their tumor clearance in vitro and in vivo.
Mooney said their findings showed that CAR-T cell products made from T cells derived from cancer patients are typically less functional than CAR-T cell products derived from healthy individuals. It could greatly improve their function to match the dose of CAR-T cell antigen stimulation to the phenotype of patient T cells using a precisely controlled biomaterial approach that closely mimics natural antigen presentation. This approach may be able to further personalize CAR-T cell therapy and eliminate the current deficiencies in T cell manufacturing.
The Key to Achieving Personalized CAR-T Cell Therapies
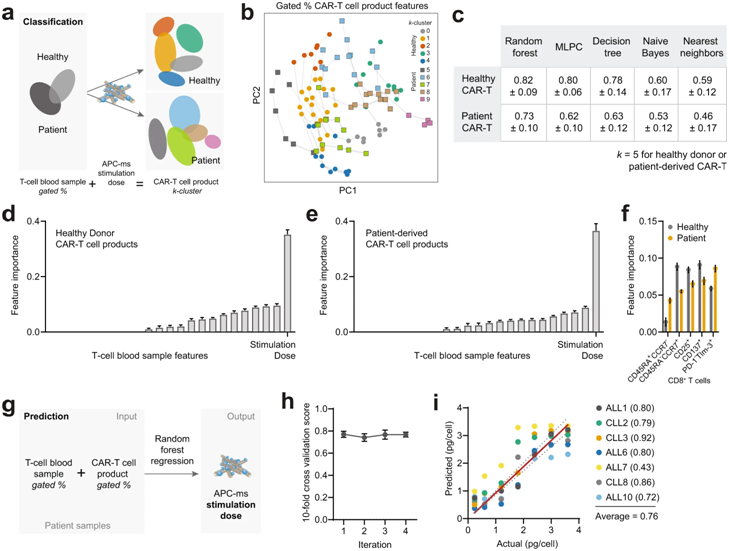
These authors studied the phenotypes of T cells they isolated from patients with acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL), as well as from healthy donors. Next, they used APC-ms to deliver different doses of anti-CD3/anti-CD28 antigen stimulation to these isolated T cells, resulting in the construction of a CAR-T cell library.
They then again tested all CAR-T cell products contained in this CAR-T cell library for functional differences, including their ability to kill cancer cells in vitro. They compared their approach directly with the method commonly used in CAR-T cell manufacturing, where the same antigens on rigid magnetic beads (called Dynabeads) are presented to T cells.
A key finding was that T cells from cancer patients were more susceptible to overstimulation than “healthy” T cells at the antigen doses commonly used in the CAR-T cell manufacturing process. CAR-T cells not only need to be transformed into a functional state, but also need to be expanded millions of times to be able to eliminate tumor cells and metastases throughout the body.
David Zhang, the first author of the paper and a graduate student in the Mooney lab, said by exploring the precise, narrow range of stimulation doses achieved by APC-ms, the team found that for patient-derived T cells, there is something like a personalized ‘sweet spot’ that allows maximizing function and expansion, which is, on average, lower than the usual dose. The APC-ms approach functions much more naturally than Dynabeads because of the highly controlled T cell signaling embedded in the lipid bilayer, which allows CAR-T cells to push and pull them, just as T cells normally do when T cell stimulation is at its optimal state by crossing the ‘immune synapse’ as they do.
From In Vitro Studies to Cell Manufacturing
Although these authors did not observe any significant differences between CAR-T cells constructed from samples from ALL patients and CLL patients, their approach, as a whole, produced more cells with high cytotoxic potential against tumor cells, a more balanced ratio of cytotoxic CD8+ T cells to the CD4+ T cells that support their function, and more memory T cells that are not cytotoxic themselves but can be activated in later responses.
In in vivo experiments in mice, infusible CAR-T cell products constructed at different stimulation levels also showed significantly different abilities to control CD19-expressing Burkitt’s lymphoma, and CAR-T cells that are stimulated again at lower-than-usual levels during manufacture showed strong cytotoxic potential.
Wu said they constructed a proof-of-concept model that is based on a quantifiable relationship between the phenotype of a T-cell blood sample and its CAR-T cell product, and outputs an optimal T-cell stimulation dose for personalized CAR-T cells. Given that T cell samples always carry important markers at the beginning of the cell manufacturing process, similar strategies can be devised to further personalize therapy using the APC-ms approach.
Reference
1. Zhang, David KY, et al. “Enhancing CAR-T cell functionality in a patient-specific manner.” Nature Communications 14.1 (2023): 506.