Next-IO™ Anti-GPC3 Therapeutic CAR-NK Cell Program
About This Program
This program aims to develop anti-GPC3 therapeutic CAR-NK cell for immuno-oncology.
Rationale when developing the program:
-
Hepatocellular carcinoma (HCC) is a common form of liver cancer that begins in hepatocytes, i.e the basic liver cell, and it accounts for 90% of primary liver cancers. One data suggests it is one of the most deadly cancers in Asia. To date, the 5-year survival rate for patients with liver cancer remains low at approximately 10%. A new but potent strategy is urgently needed for the field.
-
Glypican-3 (GPC3) is a heparan sulfate proteoglycan expressing 75% of HCC tissues but not in healthy liver or other normal tissues.
-
Chimeric antigen receptor (CAR)-modified natural killer (NK) cells, i.e GPC3-CAR NK cells, have been shown to have good results regarding its elimination activity against HCC cell lines, suggesting a new outlook in clinical translation of NK cell-based GPC3 cell therapeutics in patients with HCC.
For the reasons mentioned above, we believe GPC3 is a suitable target for CAR NK cell therapy.
GPC3
GPC3 belongs to heparin sulfate proteoglycan family, can be immobilized on the cell surface by glycosylphosphatidylinositol anchors. GPC3 can be expressed in a variety of tissues during embryonic development. Beyond that life state, it is a highly tumor-specific antigen in which expression is severely inhibited in most adult tissues.
Highlighted Functions:
-
Elevated GPC3 expression is reported in a variety of tumor types, such as liver, lung, stomach, ovarian, esophageal, and other forms of cancers, indicating GPC3 is an ideal target to be studies in patients with solid tumors.
-
In particular, GPC3 is currently used as a diagnostic biomarker to distinguish HCC from normal liver tissue, benign liver tumors, and other types of metastatic cancer.
-
There is sufficient evidence to suggest that GPC3 is involved in the malignant transformation of HCC.
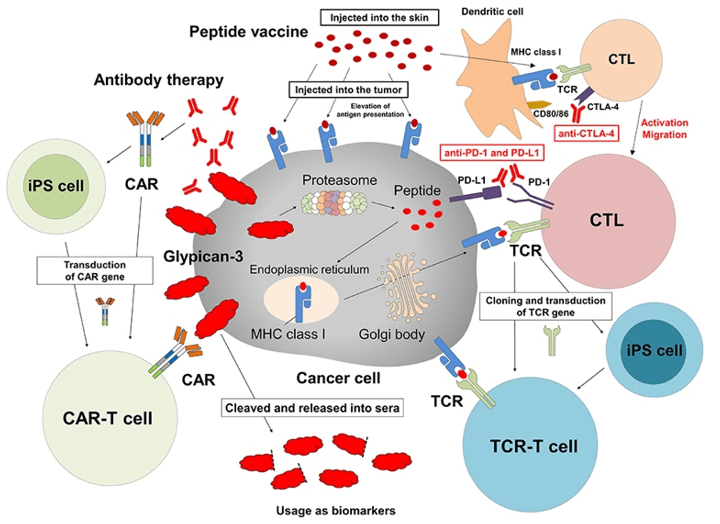 Fig.1 Cancer immunotherapy targeting GPC3. (Shimizu, 2019)
Fig.1 Cancer immunotherapy targeting GPC3. (Shimizu, 2019)
GPC3 in Cancer Studies
Here are some published data about GPC3 working as a potential target for cancer immunotherapy.
-
Anti-GPC3-CAR NK cells inhibit tumor cell growth in xenograft tumors from patients with HCC.
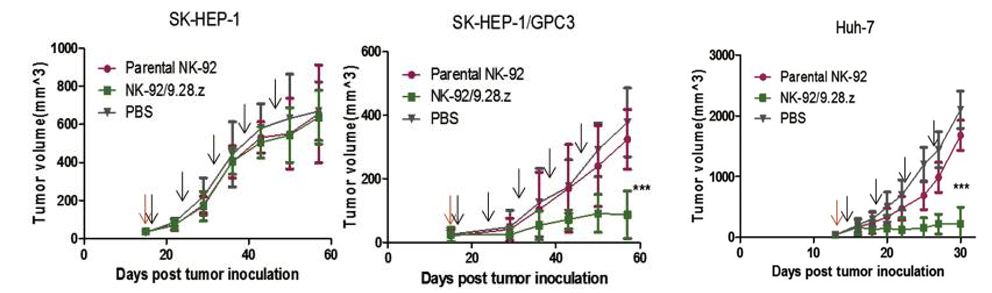 (Yu, 2018)
(Yu, 2018)
Ongoing Clinical Trials
-
Currently, NO anti-GPC3 therapeutic CAR-NK cell products are evaluated in clinical trials. The cumulative preclinical data may support GPC3 has unprecedented cancer specificity and hope to be studied in cancer immunotherapy. We believe the program will be the pioneer in the field.
-
In an effort to optimally leverage GPC3-mediated immune response, our next generation of GPC3 targeting treatment attempts to explore combination therapy trials by involving other immunomodulatory agents.
Program Planning and Management
Creative Biolabs has extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.

Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-GPC3 therapeutic CAR-NK program together. Our scientists are dedicated to bringing together years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners to advance their programs with more chance to succeed. Look forward to cooperating with you in the near future.
References
-
Shimizu, Y.; et al. Next-generation cancer immunotherapy targeting glypican-3. Frontiers in oncology. 2019, 9: 248.
-
Jiang, Z.; et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Frontiers in immunology.2017: 690.
-
Yu, M.; et al. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Molecular Therapy. 2018, 26(2): 366-378.
For Research Use Only | Not For Clinical Use


 Fig.1 Cancer immunotherapy targeting GPC3. (Shimizu, 2019)
Fig.1 Cancer immunotherapy targeting GPC3. (Shimizu, 2019)
 (Yu, 2018)
(Yu, 2018)

 Download our brochure
Download our brochure