Next-IO™ Anti-PD-L1 × 4-1BB Therapeutic Bispecific Antibody Program
About This Program
This program aims to develop PD-L1 × 4-1BB therapeutic bispecific antibody for immuno-oncology.
Currently, clinical blockade of the PD-1/L1 axis as cancer immunotherapy has shown long-lasting efficacy on patients with cancer, such as prolonged overall survival rate. However, relapsed patients do not experience the positive effects and there is an increasing need to study its effects on this group.
Activation of the tumor necrosis factor receptor (TNFR) superfamily is the key in cancer immunotherapy. From what we already knew, 4-1BB-mediated co-stimulation can support cell activation, survival, and proliferation. For this reason, we think agonist interventions with 4-1BB will become a great promise.
In summary, we propose to develop a novel bispecific antibody, PD-L1x 4-1BB. Hopefully, the end product can bind to the checkpoint of the inhibitory PD-1: PD-L1 signal axis, and is able to conditionally stimulate T cells by activating the 4-1BB receptor to enhance the immune response and allow effective cancer cells elimination.
PD-L1 × 4-1BB
The programmed death-ligand 1 (PD-L1; or CD274, B7-H1) is a key anti-phagocytic signal, i.e "don't find me" signal, to the adaptive immune system.
4-1 BB (also known as CD137) is a member of the TNFR superfamily, expressing in both innate and adaptive immune cells. By regulating tumour microenvironment (TME), CD137 can enhance anti-tumor responses and, therefore, considered as an attractive target in cancer immunotherapy.
Here are some published data regarding PD-L1 × 4-1BB work as a potential target in cancer immunotherapy.
-
Survival data for CT26.WT tumor–bearing mice treated with Ctrl, PD-L1 Mab, CD137 Mab, or a CD137/PD-L1 bispecific antibody.
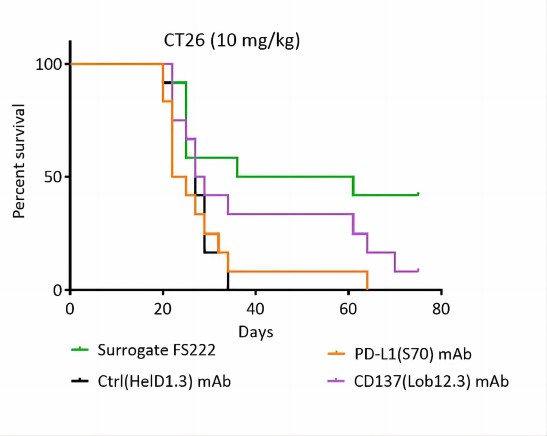 Fig.1 A CD137/PD-L1 bispecific antibody controlled tumor growth in CT26 mouse tumor models. (Lakins, et al., 2020)
Fig.1 A CD137/PD-L1 bispecific antibody controlled tumor growth in CT26 mouse tumor models. (Lakins, et al., 2020)
-
Survival data for colorectal cancer cell–bearing mice treated with Ctrl, PD-L1 Mab, CD137 Mab, or a CD137/PD-L1 bispecific antibody.
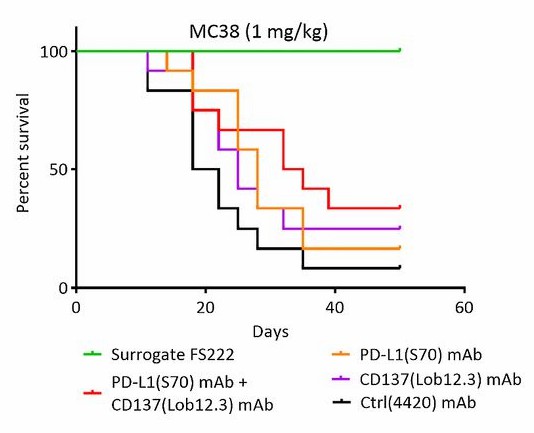 Fig.2 A CD137/PD-L1 bispecific antibody controlled tumor growth in mouse colorectal cancer models. (Lakins, et al., 2020)
Fig.2 A CD137/PD-L1 bispecific antibody controlled tumor growth in mouse colorectal cancer models. (Lakins, et al., 2020)
Ongoing Clinical Trials
-
Currently, as far as we know, only one bispecific anti-PD-L1 × 4-1BB antibody (name INBRX-105) is being evaluated in a clinical trial. Accumulated preclinical data are emerging, demonstrating the synergistic anti-tumor effects of this bispecific combination. We believe this dual-targeting strategy will provide insight into tumor immunotherapy, allowing us to fight cancer more confidently.
-
In an effort to optimally leverage PD-L1 × 4-1BB-mediated immune response, our next-generation PD-L1 × 4-1BB targeted antibody program attempts to explore the optimal combination strategy - that is, how to exert the best anti-tumor effect when PD-L1 × 4-1BB is synergistically expressed.
Program Planning and Management
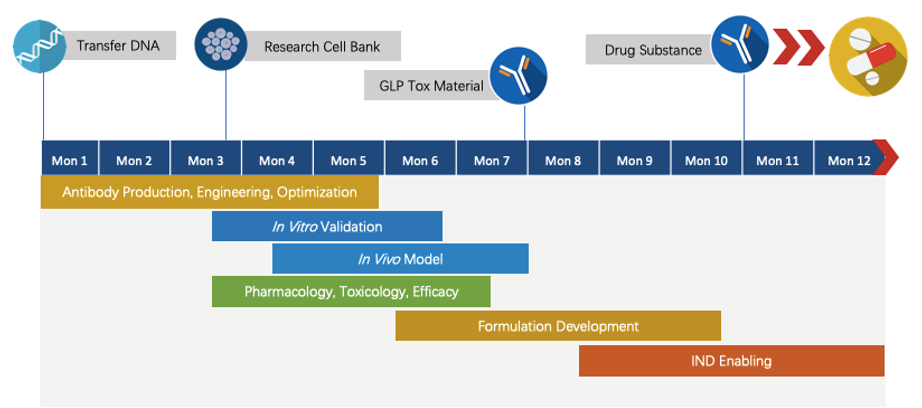
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs focuses on discovering and developing innovative monoclonal antibody-based therapies for cancer treatment. We are looking for potential partners (include but not limit to major pharma or biotech firms) to develop PD-L1 × 4-1BB bispecific antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners advance their programs with more chance to proceed. Look forward to cooperating with you in the near future.
References
-
Liu, X.; et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell reports. 2018, 24(8): 2101-2111.
-
Lakins, M.A.; et al. FS222, a CD137/PD-L1 tetravalent bispecific antibody, exhibits low toxicity and antitumor activity in colorectal cancer models. Clinical Cancer Research. 2020, 26(15): 4154-4167.
For Research Use Only | Not For Clinical Use


 Fig.1 A CD137/PD-L1 bispecific antibody controlled tumor growth in CT26 mouse tumor models. (Lakins, et al., 2020)
Fig.1 A CD137/PD-L1 bispecific antibody controlled tumor growth in CT26 mouse tumor models. (Lakins, et al., 2020)
 Fig.2 A CD137/PD-L1 bispecific antibody controlled tumor growth in mouse colorectal cancer models. (Lakins, et al., 2020)
Fig.2 A CD137/PD-L1 bispecific antibody controlled tumor growth in mouse colorectal cancer models. (Lakins, et al., 2020)

 Download our brochure
Download our brochure