Next-IO™ Anti-TIGIT Monoclonal Antibody Program
As a thriving CRO company, Creative Biolabs has initiatives to acquire, develop and commercialize Next-IO™ programs for therapeutic purposes. Our anti-TIGIT monoclonal antibody program aims to develop therapeutic monoclonal antibodies that could recognize and potentially, against T-cell Ig, and ITIM domain (TIGIT).
Background
Inhibitory receptors are critical in maintaining immune homeostasis. Because the fact that upregulation of inhibitory receptors can hinder T cell responses, therapies target on inhibitory receptors are currently at the forefront of cancer treatments. In recent years, antibodies targeting inhibitory receptors, PD1 and CTLA4, have shown promising results in various cancers. However, because of the individual difference, not all cancer patients benefit from these drugs and enormous interests are on the therapies targeting other immune checkpoints. Our program focuses on novel cancer immunotherapies targeting TIGIT, which is an inhibitory immune checkpoint that plays an important role in immune suppression.
Mechanism of Action
T cell Ig and ITIM domain (TIGIT) is expressed on the surface of a variety of lymphoid cells, including T cells, natural killer (NK) cells, and regulatory T cells (Tregs). Several studies have demonstrated that TIGIT marks the most dysfunctional subset of CD8 positive T cells and Tregs with a highly suppressive phenotype in various cases. In addition, the blockade of TIGIT exhibits therapeutic benefits in different animal tumor models.
This program will only design monoclonal antibody that binds to TIGIT, specifically. We understand anti-TIGIT shows better anti-tumor efficacy when working with anti-PD1. For combination therapies, please reach out to our scientists for additional assistance.
Published Data
Based on these data, we can learn anti-TIGIT exhibit promising therapeutic benefits for a variety of diseases including glioblastoma multiforme, Acute myeloid leukemia, HIV infection, ALS, etc.
Clinical Trials
So far, monoclonal antibodies developed against programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have all shown some level of anti-tumor efficacy, and several antibodies have already approved by officials to use in practice. TIGIT, as a key immune checkpoint, is currently being evaluated in many studies and clinical trials. Till today, not one drug anti-TIGIT is approved. We believe our program of discovering and researching on anti-TIGIT is at the cutting edge of the field and have certain marking and sale potential.
Program Planning and Management
We have extensive experience in performing comprehensive program developments and effective problem-solving. For our Next-IO™ programs, we are committed to delivering the finalized program to our partners within about 1.5 years. The accurate timeline will be determined on a case-by-case basis. Here is a draft timeline for your glance.
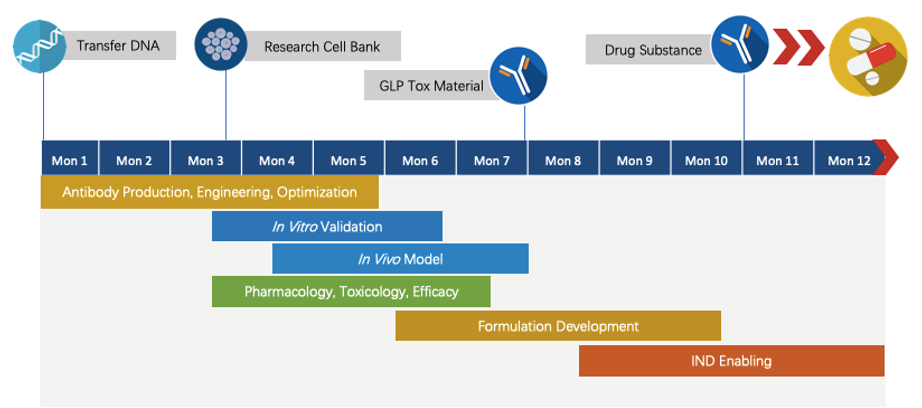 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
Collaboration
Creative Biolabs is looking for partners to help us to gain a competitive edge in the field and maximize the impacts of the programs. We have developed Next-IO™ programs specifically to fit your desired direction. With your support and collaboration, we are dedicated to developing novel cancer immunotherapies that can hope bother parties to achieve greater success.
If you are interested in our programs, please feel free to contact us for more details.
Reference
-
Song, Yangzi, et al. "T‐cell Immunoglobulin and ITIM Domain Contributes to CD 8+ T‐cell Immunosenescence." Aging cell 17.2 (2018): e12716.
For Research Use Only | Not For Clinical Use


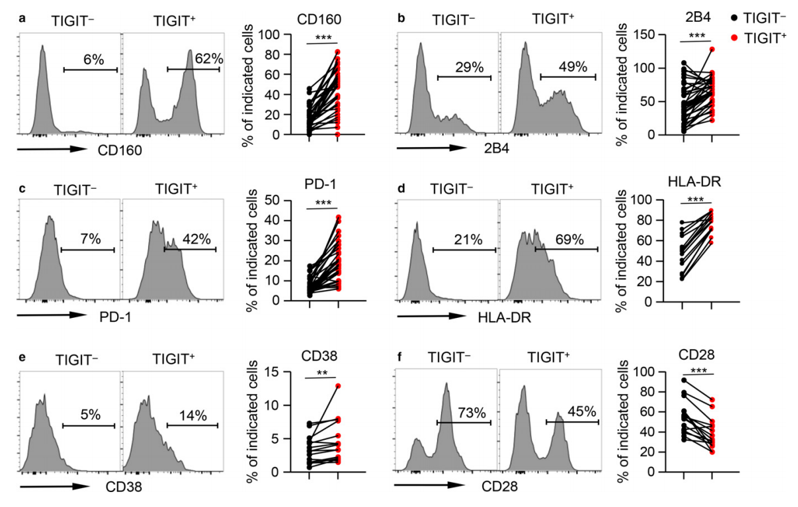 Fig.1 TIGIT expression is associated with exhaustion and overactivation of inhibitory phenotypes.1
Fig.1 TIGIT expression is associated with exhaustion and overactivation of inhibitory phenotypes.1
 Fig.2 The timeline of Next-IOᵀᴹ programs.
Fig.2 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure