Antimicrobial Susceptibility Test (AST) Service
Creative Biolabs provides a clear, data-driven pathway to validate your product's efficacy. Our services are designed to deliver actionable intelligence and a clear competitive advantage by moving your product from the lab bench to the market faster and with greater confidence. By providing this rigorous scientific foundation, we help you mitigate risk, reduce the time and cost associated with development, and build a compelling case for your product's value to regulators and end-users.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
What is Antimicrobial Susceptibility Testing
Antimicrobial Susceptibility Testing (AST) is a critical discipline for evaluating the effectiveness of antimicrobial agents against drug-resistant microorganisms. This process employs a range of standardized and advanced techniques to determine a compound's potency, speed of action, and mechanism of action. By providing a clear, evidence-based assessment of a product's performance, this testing reduces development risk and informs effective public health strategies.
To understand how this service can be leveraged to address specific project requirements, request a consultation.
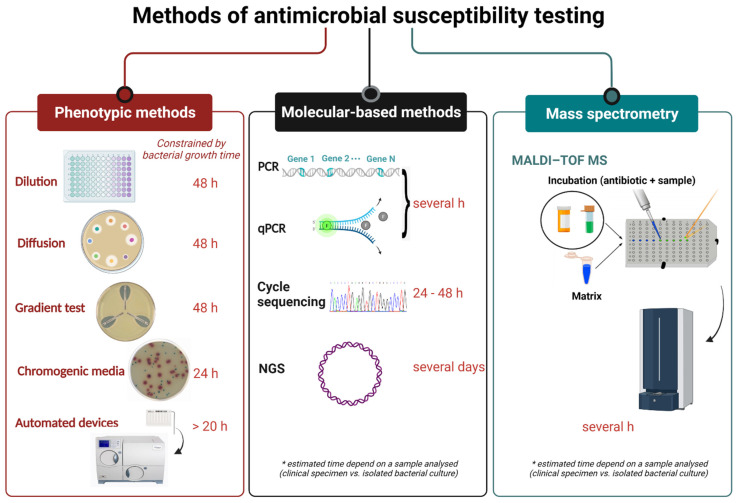 Fig.1 Current methods for antimicrobial susceptibility testing and turnaround time.1
Fig.1 Current methods for antimicrobial susceptibility testing and turnaround time.1
What We Can Offer
Phenotypic Testing
We use traditional, gold-standard methods like the Kirby-Bauer disc diffusion and broth microdilution assays to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of your product.
Genotypic Testing
Our molecular biology services, including PCR and Whole Genome Sequencing, identify specific genes responsible for antimicrobial resistance, providing insight into the mechanism and future resistance potential.
Rapid-Testing Methods
Our cutting-edge, metabolism-based assays, like ATP bioluminescence, provide rapid results in hours, accelerating your screening process and allowing for quicker go/no-go decisions.
Surface and Material Efficacy
We conduct real-world simulations to test the efficacy of your product on various surfaces and materials, essential for medical devices and disinfectants.
Custom Challenge Testing
We design and execute bespoke test protocols tailored to your unique product and target pathogens, providing comprehensive and highly relevant data for your specific needs.
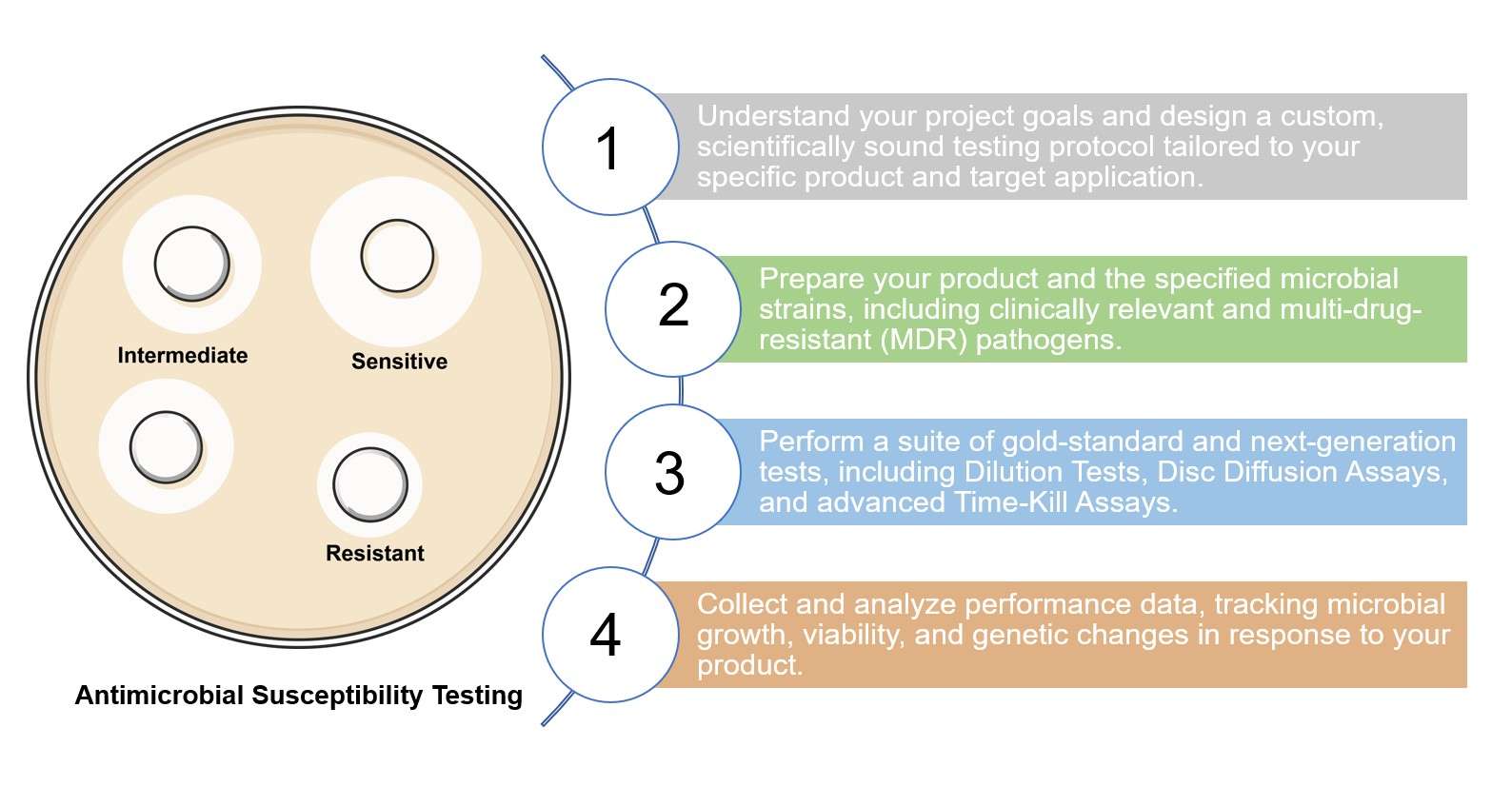
Workflow of AST Service at Creative Biolabs
Highlights
Scientific Expertise
Our team of expert microbiologists and geneticists possesses extensive experience across both traditional and cutting-edge AST methodologies. We bring deep scientific insight to every project, ensuring your data is accurate and scientifically sound for any application.
Unmatched Efficiency
By leveraging our rapid-testing platforms and streamlined workflows, we drastically cut down the time required to generate critical data. This efficiency translates directly into faster development cycles and a reduced time-to-market for your product, saving valuable resources.
Comprehensive Approach
We offer a hybrid service model that combines gold-standard phenotypic assays with advanced genotypic analysis. This comprehensive approach provides a complete picture of your product's efficacy, helping you understand both its immediate impact and its long-term resistance profile.
Dedicated Partnership
We offer continuous expert consultation, from initial protocol design to final report interpretation. Our goal is to ensure you have all the information and support needed to succeed.
For a detailed assessment of our capabilities and a tailored cost estimate - Get a quote today.
Customer Reviews
-
Optimized Product Formulation
Using Creative Biolabs' service, we were able to quickly pinpoint the most effective concentration for our new disinfectant, saving us months of trial and error. Their expertise in both phenotypic and genotypic testing is unmatched. - S***y T.
-
Precise Mechanism of Action Insights
Creative Biolabs' genotypic analysis provided us with a deeper understanding of our compound's mechanism of action. This level of detail was instrumental in refining our development strategy and strengthening our patent application. - B***e G.
FAQs
How do your rapid testing methods compare to traditional assays?
Our rapid methods, such as ATP bioluminescence, offer results in hours rather than days, significantly accelerating your development cycle. While traditional methods remain the gold standard for regulatory submissions, our rapid tests are perfect for early-stage screening and for quickly eliminating ineffective compounds.
Is your testing suitable for my specific product type (e.g., medical device, disinfectant, pharmaceutical)?
Absolutely. We customize every testing protocol to the specific needs of your product. Our lab is equipped to simulate real-world conditions for medical devices and disinfectants, and we have deep expertise in the requirements for pharmaceutical compounds.
Related Services
Microbial Enumeration Test
We perform quantitative counts of microorganisms in a sample, a key requirement for product safety and quality control testing across various industries.
Learn More →
Antimicrobial Effectiveness Test (AET)
This service evaluates a product's ability to withstand microbial contamination during use, as mandated by pharmacopeial guidelines for multi-use products like cosmetics and topical drugs.
Learn More →
How to Contact Us
The fight against AST requires a proactive and precise approach. Partner with Creative Biolabs to ensure your product has a meaningful impact on global health. Our expert team is ready to assist you. For more information and to discuss your project, please contact us.
Reference
-
Gajic, Ina et al. "Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods." Antibiotics (Basel, Switzerland) vol. 11,4 427. 23 Mar. 2022. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/antibiotics11040427
For Research Use Only | Not For Clinical Use


 Fig.1 Current methods for antimicrobial susceptibility testing and turnaround time.1
Fig.1 Current methods for antimicrobial susceptibility testing and turnaround time.1


