Next-IO™ CD22 × CD19 Therapeutic CAR-T Program
About This Program
This program aims to develop CD22 × CD19 therapeutic CAR-T for B cell acute lymphoblastic leukemia (B-ALL).
Rationale for our program:
-
CD19-directed chimeric antigen receptor T (CAR-T) cells showed an unprecedented initial response rate in relapsed/refractory (R/R) B cell acute lymphoblastic leukemia (B-ALL). However, recurrence due to loss or down-regulation of CD19 poses a new threat to this innovative form of cellular immunotherapy.
-
CAR-T cells specific for CD22 (another B cell antigen lineage) also showed comparable potency to CD19-directed CAR-T cells in 21 adult B-ALL patients.
-
CAR-T cells that simultaneously target CD19 and CD22 have been shown to overcome the potential benefits of CD19 immune escape, and early clinical experience in this approach in pediatric and adult B cell malignancies has shown encouraging results.
Given the above, the novel bispecific CAR targeting CD19 and CD22 we proposed might be an attractive strategy to address the recurrence of antigen escape after CD19-directed CAR-T cell therapy.
CD22 Emerges as CAR T-Cell Therapy Target
CD22 is a B cell-restricted phosphoglycoprotein of the immunoglobulin superfamily and has attracted widespread attention as a therapeutic target for B cell-directed therapy. Several novel therapeutic agents that selectively target CD22 are being developed as an alternative to B cell cancer therapy.
CD22 is expressed in almost all lymphoid malignancies. It has been reported that CD22 expression among different subtypes of ALL is 83% of Pro-B, 96.4% of normal B cells, 91.9% of Pre-B and 100% of mature B cell ALL. In several subtypes of mature B cell lymphoma, CD22 expression is reported to be higher than 90%. Therefore, CD22 can be used as a potent target for the treatment of B cell malignancies.
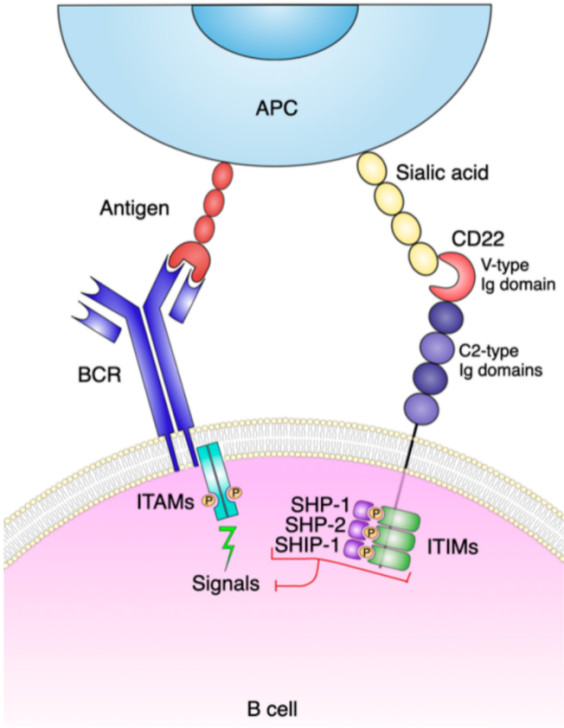 Fig.1 CD22 structure and signaling pathway.1,3
Fig.1 CD22 structure and signaling pathway.1,3
Supporting Data
The following data support the rationale for the development of CD22 × CD19 bispecific CAR-T cells with an improved therapeutic index in B-ALL.
-
The CAR simultaneously targeting CD19 and CD22 has the potential of inducing long-term remission in patients with B-ALL.
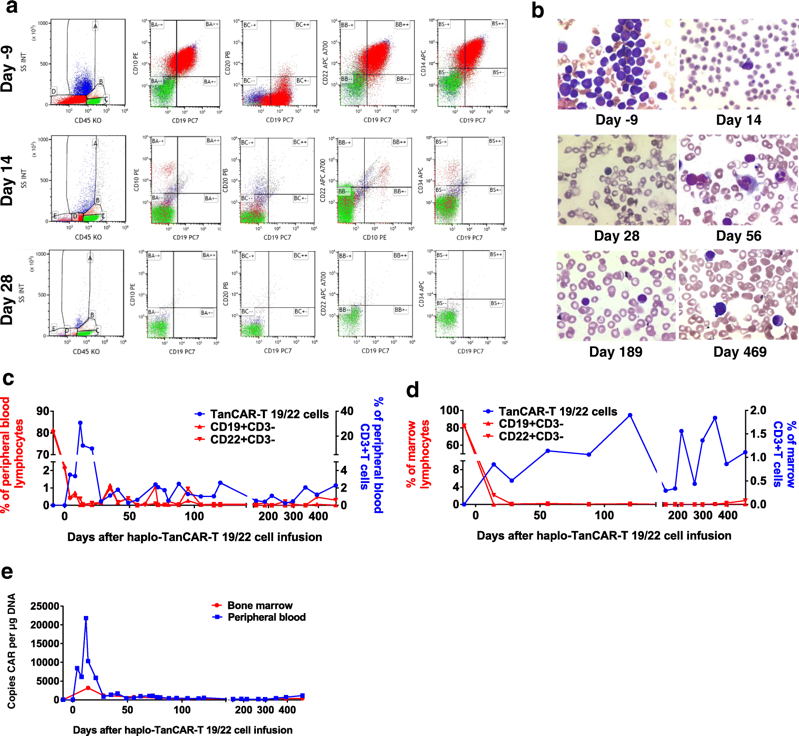 Fig.2 Clinical activity and expansion of TanCAR-T 19/22 cells.2,3
Fig.2 Clinical activity and expansion of TanCAR-T 19/22 cells.2,3
Ongoing Clinical Trials
-
Currently, only two CD19/CD22 CAR cell products are being evaluated in clinical phase 1 trial. The cumulative preclinical data are emerging to support that the combination of CD19/CD22 has unprecedented cancer specificity.
|
NCT ID
|
Status
|
Sponsor
|
Lead Sponsor
|
Phase
|
Update Time
|
|
NCT03241940
|
Recruiting
|
Crystal Mackall, MD
|
Phase I CD19/CD22 Chimeric Antigen Receptor T Cells in Peds Recurrent/Refractory B Cell Malignancies
|
Phase 1
|
August 29, 2019
|
|
NCT03448393
|
Recruiting
|
National Cancer Institute (NCI)
|
CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory CD19/CD22-expressing B Cell Malignancies
|
Phase 1
|
July 19, 2019
|
-
We believe the dual-targeting strategy can provide further insights into tumor immunotherapy, which allows us to fight cancer more efficient. In an effort to optimally leverage CD22 × CD19-mediated immune response, our next-generation CD22 × CD19 targeted antibody program attempts to explore the optimal combination strategy - that is, how to exert the best anti-tumor outcome while synergistically produce CD22 × PD-L1.
Program Management
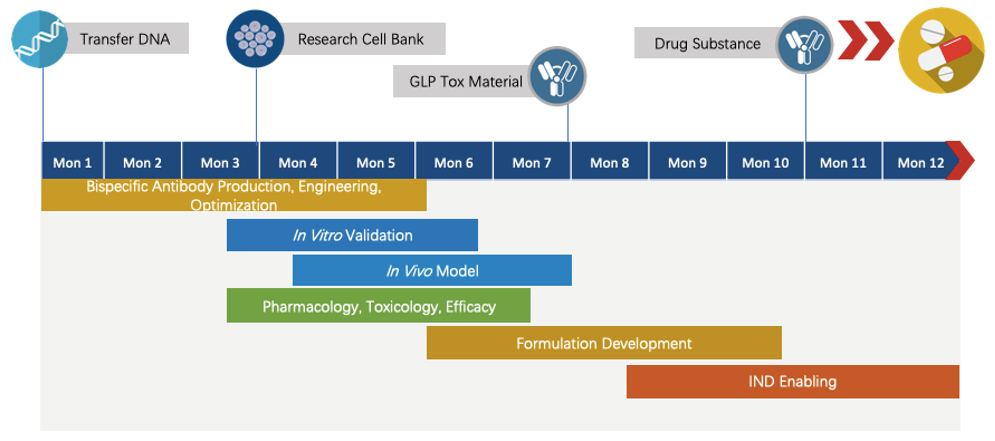
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop CD22 × CD19 therapeutic CAR-T program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
References
-
Lanza, Francesco, et al. "CD22 expression in B-cell acute lymphoblastic leukemia: biological significance and implications for inotuzumab therapy in adults." Cancers 12.2 (2020): 303.
-
Jia, Hejin, et al. "Haploidentical CD19/CD22 bispecific CAR-T cells induced MRD-negative remission in a patient with relapsed and refractory adult B-ALL after haploidentical hematopoietic stem cell transplantation." Journal of hematology & oncology 12 (2019): 1-9.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 CD22 structure and signaling pathway.1,3
Fig.1 CD22 structure and signaling pathway.1,3
 Fig.2 Clinical activity and expansion of TanCAR-T 19/22 cells.2,3
Fig.2 Clinical activity and expansion of TanCAR-T 19/22 cells.2,3

 Download our brochure
Download our brochure