Next-IO™ CTLA-4 x OX40 Therapeutic Bispecific Antibody Program
About This Program
This program aims to develop CTLA-4 x OX40 therapeutic bispecific antibody for bladder carcinoma immunotherapy.
Rationale for our program:
-
The approval of the anti-CTLA-4 antibody ipilimumab (Yervoy®) in 2011 revolutionized the field of immuno-oncology (IO) by significantly improving the long-term survival of patients with metastatic melanoma. However, CTLA-4 blocking antibodies are associated with severe immune-related adverse events caused by systemic activation of T cells, which limits the use of CTLA-4 mAb.
-
The next generation of CTLA-4 targeted therapies, checkpoint inhibitors and T cell co-stimulatory agonistic antibody combinations have been proposed. It has a dual mode of action that promotes the activation of effector T cells in tumors while controlling Treg inhibition, which converts cold tumors into hot tumors.
-
In several types of cancer, both CTLA-4 and OX40 are highly upregulated in tumor invasive Tregs.
CTLA-4 x OX40
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, CD152), an immune checkpoint, is a membrane glycoprotein involving in the suppression of T cell immune functions like proliferation and cytokine production. CTLA-4 is a negative regulator of anti-tumor T cells upon recognition of their ligands. Currently, therapeutic antibodies targeting CTLA-4, ipilimumab, and tremelimumab, are approved to be efficient in a wide range of hematological malignancies and solid tumors.
OX40 is a costimulatory receptor on the surface of T cells that regulates costimulatory signals. Currently, anti-OX40 monoclonal antibodies are used as monotherapy or in combination with checkpoint inhibitors in clinical development. However, with the exception of Alligator's ATOR-1015, there are no other bsAb targeting checkpoint inhibitors and T cell costimulatory receptors in clinical development.
Supporting Data
The following data support the rationale for the development of CTLA-4 x OX40 BiAbs with an improved therapeutic index for the treatment of bladder cancer.
-
The CTLA-4 x OX40 bispecific antibody (ATOR-1015) induced a significantly anti-tumor effect in a bladder cancer model.
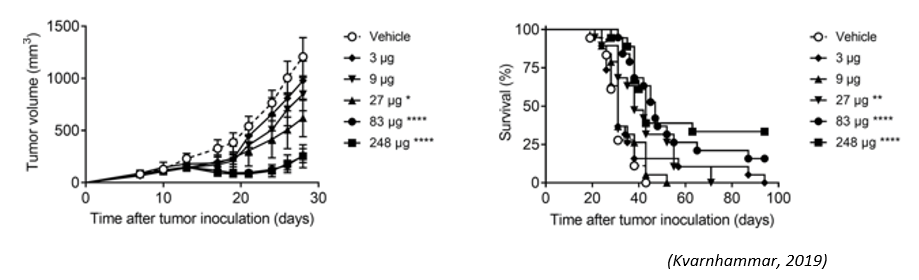 Fig.1 The anti-tumor effect of CTLA-4 x OX40 bispecific antibody.1,2
Fig.1 The anti-tumor effect of CTLA-4 x OX40 bispecific antibody.1,2
Bladder Cancer
-
Bladder cancer (BC) is one of the most common malignant tumors of the genitourinary system, and despite the established risk factors such as age, smoking, and family history, there is a lack of early detection strategies.
-
BC is the ninth most common malignancy in the world, with approximately 2.5 million patients and 420,000 newly diagnosed cases each year.
-
The global market for BC is estimated at $855 million in 2018 and is growing exponentially, with a compound annual growth rate of 12.5% till 2023.
Ongoing Clinical Trials
-
Currently, only one biotechnology company (Pieris Pharmaceuticals, Inc.) presented their preclinical data on the CTLA-4 x OX40 BsAb in April 2019. Therefore, NO project on CTLA-4 x OX40 BsAbs has entered clinical research.
-
We believe that this dual targeting strategy will provide insights into the tumor immunotherapy, especially in the treatment of BC. In an effort to optimally leverage CTLA-4 x OX40-mediated immune response, our Next-IO™ CTLA-4 x OX40 targeted antibody program attempts to explore the optimal combination strategy - that is, how to exert the best anti-tumor outcome while synergistically produce CTLA-4 and OX40.
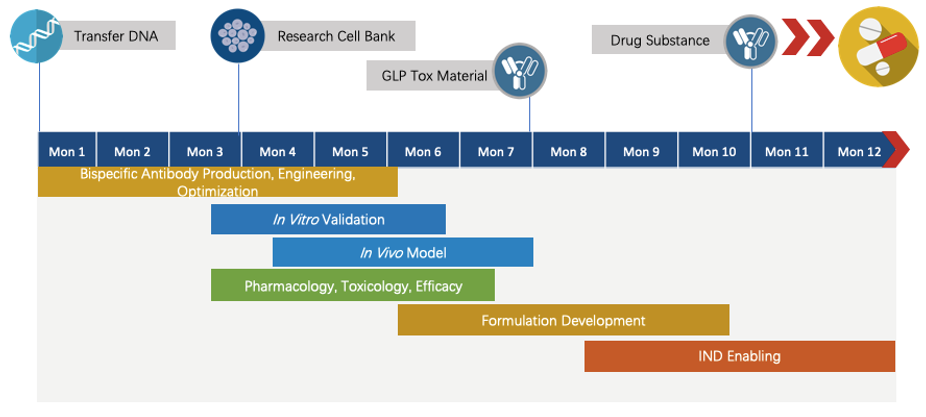
Program Plan
Creative Biolabs has extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop CTLA-4 x OX40 therapeutic bispecific antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
Reference
-
Kvarnhammar, Anne Mansson, et al. "The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation." Journal for immunotherapy of cancer 7 (2019): 1-14. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 The anti-tumor effect of CTLA-4 x OX40 bispecific antibody.1,2
Fig.1 The anti-tumor effect of CTLA-4 x OX40 bispecific antibody.1,2

 Download our brochure
Download our brochure